中国组织工程研究 ›› 2021, Vol. 25 ›› Issue (19): 3049-3056.doi: 10.3969/j.issn.2095-4344.3501
• 干细胞综述 stem cell review • 上一篇 下一篇
干细胞治疗多发性硬化及视神经脊髓炎谱系疾病的有效性和临床应用限制
张红庆,谢旭芳,吴晓牧
- 南昌大学第一附属医院神经内科,江西省南昌市 330000
-
收稿日期:2020-04-11修回日期:2020-04-25接受日期:2020-05-30出版日期:2021-07-09发布日期:2021-01-13 -
通讯作者:吴晓牧,博士,教授,主任医师,博士生导师,南昌大学第一附属医院神经内科,江西省南昌市 330000 -
作者简介:张红庆,女,1995年生,江西省新余市人,汉族,南昌大学医学院在读硕士,主要从事神经免疫性疾病的研究。 -
基金资助:江西省重点研发项目(20171ACH80001),项目负责人:吴晓牧;江西省卫健委资助项目(2018A026),项目负责人:谢旭芳
Effectiveness and clinical application limitations of stem cells in the treatment of multiple sclerosis and optic neuromyelitis spectrum diseases
Zhang Hongqing, Xie Xufang, Wu Xiaomu
- Department of Neurology, First Affiliated Hospital of Nanchang University, Nanchang 330000, Jiangxi Province, China
-
Received:2020-04-11Revised:2020-04-25Accepted:2020-05-30Online:2021-07-09Published:2021-01-13 -
Contact:Wu Xiaomu, MD, Professor, Chief physician, Doctoral supervisor, Department of Neurology, First Affiliated Hospital of Nanchang University, Nanchang 330000, Jiangxi Province, China -
About author:Zhang Hongqing, Master candidate, Department of Neurology, First Affiliated Hospital of Nanchang University, Nanchang 330000, Jiangxi Province, China -
Supported by:the Key Research and Development Project of Jiangxi Province, No. 20171ACH80001 (to WXM); the Project supported by Jiangxi Health and Health Commission, No. 2018A026 (to XXF)
摘要:
文题释义:
脱髓鞘疾病:髓鞘是包裹在神经细胞轴突外面的一层膜,其绝缘作用可以防止神经电冲动从神经元轴突传递至另一神经元轴突。脱髓鞘是指髓鞘形成后发生的髓鞘损坏,脱髓鞘疾病是以神经髓鞘脱失为主,神经元胞体及轴突相对受累较轻为特征的一组疾病。多发性硬化及视神经脊髓炎谱系疾病都属于中枢神经系统脱髓鞘疾病。
干细胞治疗中枢神经系统脱髓鞘疾病的价值:近年来,干细胞在一些自身免疫性疾病中的应用越来越广泛。由于干细胞对受损的神经组织、轴突等具有保护及修复作用,使其在治疗诸如多发性硬化、视神经脊髓炎谱系疾病等脱髓鞘疾病中的应用受到大家的青睐。
背景:干细胞的自我更新及分化潜能使其成为医学领域中的研究热点,其免疫调节、神经保护等作用使干细胞在多发性硬化、视神经脊髓炎谱系疾病中的应用受到临床医生的青睐。
目的:总结近几年来干细胞治疗多发性硬化及视神经脊髓炎谱系疾病的研究进展。
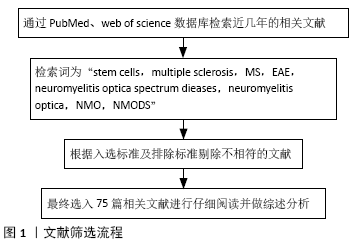
方法:通过检索PubMed、Web of science数据库中近几年发表的与干细胞治疗多发性硬化及视神经脊髓炎谱系疾病相关的文献,检索词为“stem cells,multiple sclerosis,MS,EAE,neuromyelitis optica spectrum diseases,neuromyelitis optica,NMO,NMODS”,根据选入标准及排除标准,最终纳入75篇相关文献进行仔细阅读并做综述分析。
结果与结论:与过去的免疫抑制疗法相比,干细胞移植治疗多发性硬化及视神经脊髓炎谱系疾病具有很大的潜力。但关于细胞的选择、供体的来源、移植的时间、途径、移植后长期的安全性及有效性等问题限制了临床的应用。
https://orcid.org/0000-0003-0333-2812(张红庆)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
张红庆, 谢旭芳, 吴晓牧. 干细胞治疗多发性硬化及视神经脊髓炎谱系疾病的有效性和临床应用限制[J]. 中国组织工程研究, 2021, 25(19): 3049-3056.
Zhang Hongqing, Xie Xufang, Wu Xiaomu. Effectiveness and clinical application limitations of stem cells in the treatment of multiple sclerosis and optic neuromyelitis spectrum diseases[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 3049-3056.
尽管目前有很多种药物均可用于多发性硬化的治疗,但其大多数只能控制病情的进展,改善患者的临床症状,却无法彻底治愈该疾病。患者的临床表现一旦发展为进行性残疾,就没有有效的方法保护、修复及再生神经组织以恢复患者的神经功能,因此髓鞘及神经细胞再生问题仍然是治疗多发性硬化的主要障碍[14-15]。在过去的20年中,干细胞移植被认为是侵袭性多发性硬化的潜在有效治疗方法[16],而不同类型的干细胞,甚至是相同类型但不同来源的干细胞都具有其独特的特征。
2.1.1 造血干细胞移植治疗多发性硬化 造血干细胞移植根据供者的来源可分为自体造血干细胞移植和异基因造血干细胞移植。由于自体造血干细胞移植导致的移植相关死亡率更低,因而更常用于治疗多发性硬化等一些免疫性疾病[17]。尽管在一些临床试验中可以观察到自体造血干细胞移植治疗多发性硬化的效果,但其具体的治疗机制仍不清晰。人们普遍认为,自体造血干细胞移植治疗多发性硬化是通过输注造血干细胞清除患者体内存在故障的免疫系统,重新建立机体的免疫系统,恢复其免疫耐受及免疫诱导作用,抑制患者自身的免疫反应。患者免疫耐受作用的恢复可能是由调节性T细胞及程序性死亡受体1抑制性信号转导所调节,调节性T细胞可抑制Th17驱动的促炎和自身免疫反应,并减少自身反应性T细胞的形成[18-20]。自体造血干细胞移植后,多发性硬化患者外周血中的Th17及Th1活性明显降低,而自然杀伤细胞的重塑能力增强,自然杀伤细胞与CD4+T细胞比值增
加[21-22]。通过微阵列DNA芯片技术发现,自体造血干细胞移植可以改变外周CD4+和CD8+T细胞亚群的基因表达,而在移植2年后多发性硬化患者的CD8+T细胞簇的表达与健康对照组相似[23]。ZEHER等[20]研究也表明多发性硬化患者外周血中CD8+T细胞含量在移植3个月后可恢复正常,B淋巴细胞在第6个月后恢复至正常水平。
造血干细胞移植的第一步包括动员体内的造血干细胞,目前常用的动员方案包括使用粒细胞集落刺激因子或粒细胞-巨噬细胞集落刺激因子或粒细胞集落刺激因子/粒细胞-巨噬细胞集落刺激因子+环磷酰胺方案,RADAELLI等[24]在早期的研究中已经证实,粒细胞集落刺激因子/粒细胞-巨噬细胞集落刺激因子+环磷酰胺方案比单用粒细胞集落刺激因子或粒细胞-巨噬细胞集落刺激因子的效果更佳。在造血干细胞回输前,患者需使用一些化学药物及免疫抑制剂以防移植后体内自身反应性淋巴细胞的扩增,目前常用的调节方案多为以BEAM(卡马汀、依托泊苷、阿糖胞苷、美法仑)为主的中强度调节方案[2]。在通过BEAM-抗免疫球蛋白对多发性硬化患者进行预处理,患者接受造血干细胞移植治疗后4年和5年的无病生存率分别可达到73.8%和69.2%,但也有报道显示患者在使用高强度调节方案进行预处理后,在长达13年的随访时间内并未观察到疾病的进展[19,25]。另外,尽管自体造血干细胞移植可以有效降低多发性硬化复发率,但其可能不会阻止疾病晚期的残疾进展[26]。因此,选择合适的移植时间或是在移植前对患者预处理,亦或是移植前的动员方案对造血干细胞的移植效果都至关重要。
根据欧洲血液和骨髓移植学会上登记的数据表明[27],目前已有超过1 446例多发性硬化患者接受了自体造血干细胞移植治疗。在2012至2016年接受自体造血干细胞移植的多发性硬化患者的移植相关死亡率为0.2%,而70%-80%的复发缓解型多发性硬化患者在造血干细胞移植后的四五年内病情都趋于稳定状态[28]。在一些临床研究中,多以扩展残疾状况量表(Expanded Disability Status Scale,EDSS)在移植前后的变化、影像学检查的改变或无进展生存率来评估造血干细胞移植治疗多发性硬化的安全性及治疗效果[29]。
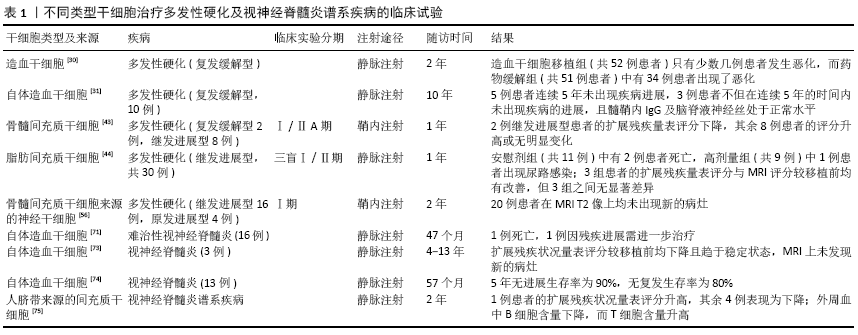
BURT等[30]在2005至2016年期间共招募了110名复发缓解型多发性硬化患者进行一项随机对照试验,这110名患者被随机分配到药物缓解治疗组或接受非清髓性(环磷酰胺200 mg/kg+抗免疫球蛋白6 mg/kg)造血干细胞移植治疗组,最终造血干细胞移植组共有52例患者接受随访,药物缓解治疗组有51例患者接受随访。在平均2年的随访期间内,造血干细胞移植组只有少数几例患者的临床症状或影像学检查发生了恶化,而药物缓解治疗组却有34例患者表现为疾病进展;造血干细胞移植组的扩展残疾状况量表评分在1年后由3.38分降至2.36分,而药物缓解治疗组从3.31分升高至3.98分;在影像学方面,造血干细胞移植组MRI T2加权像上的平均病变体积在1年时减少了31.7%,而药物缓解治疗组1年后增加了34.3%。在瑞典,TOLF等[31]对10例复发缓解型多发性硬化患者接受自体造血干细胞移植治疗后进行了10年的随访,并将持续5年未出现疾病进展定义为多发性硬化持续完全缓解;在此基础上,若髓鞘内IgG及脑脊液神经丝处于正常水平则视为多发性硬化“已治愈”。在随访结束后,其中有5例患者达到完全缓解的标准,3例患者达到“治愈”的标准。
2.1.2 间充质干细胞移植治疗多发性硬化 间充质干细胞是一种易于从组织中分离的干细胞,其来源比造血干细胞更广泛,除骨髓外,脂肪、胚胎组织、脐血等均可作为间充质干细胞的来源[32]。CD105、CD166、CD73、CD90、CD29、Octamer 4、STRO-1被视为间充质干细胞的阳性标记物,而CD34、CD45、CD14、HLA-DR被认为是其阴性标记物[33]。
间充质干细胞主要通过调节免疫反应及促进神经修复发挥其对多发性硬化的治疗作用,其免疫调节作用表现为:抑制先天性和适应性免疫应答、抑制致病性效应CD4+T细胞及B细胞的增殖、调节CD8+T细胞亚群、诱导调节性T细胞的生成、影响树突状细胞及自然杀伤细胞的功能;而神经修复功能则是通过分泌多种神经营养因子、影响神经干细胞分化、促进髓鞘再生和轴突存活所产生[3,16,34]。
BARATI等[35]在双环己酮草酰二腙诱导的脱髓鞘动物模型研究中证实,促进M2型小胶质细胞的生成以及抑制促炎细胞因子的表达可能是间充质干细胞治疗脱髓鞘疾病的机制。骨髓间充质干细胞通过抑制中枢神经系统内的炎症反应,调节白细胞介素6的表达,刺激神经生长因子的生成,保护轴突等作用改善多发性硬化患者的症状[36]。WANG等[37]研究表明骨髓间充质干细胞上清液可通过B220+B细胞影响CD4+T细胞的功能,抑制实验性自身免疫性脑脊髓炎外周血中炎性因子的分泌,减少实验性自身免疫性脑脊髓炎小鼠中枢神经系统内脱髓鞘病变的程度。与人骨髓间充质干细胞相比,人胚胎干细胞来源间充质干细胞可显著降低实验性自身免疫性脑脊髓炎的临床症状并能更有效阻止脱髓鞘作用,而造成这种差异可能与人胚胎干细胞来源间充质干细胞的高渗透能力有关[37]。除骨髓间充质干细胞外,脂肪间充质干细胞也常用于治疗多发性硬化及实验性自身免疫性脑脊髓炎。脂肪间充质干细胞可通过血脑屏障,减少大脑B细胞、T细胞及巨噬细胞的浸润,在用脂肪间充质干细胞治疗的实验性自身免疫性脑脊髓炎小鼠中,人白细胞抗原G是减轻疾病严重程度的主要因素之一[38]。此外,LI等[39]研究发现脂肪间充质干细胞还可以通过释放白血病抑制因子降低Th17/Treg比率,减轻实验性自身免疫性脑脊髓炎残疾程度。
KURTE及其同事[40]观察到间充质干细胞在实验性自身免疫性脑脊髓炎小鼠疾病发作之前或在疾病高峰期时移植的治疗效果最佳,而SHALABY等[38]在之后的研究中也证实了在疾病高峰期将脂肪间充质干细胞注射至实验性自身免疫性脑脊髓炎小鼠体内比在疾病稳定后注射的治疗效果更好,这表明间充质干细胞移植治疗的有效性与其移植时间有很大的关联。STRONG等[41]研究强调了选择供体的重要性,研究人员通过腹腔注射的方式,将来自肥胖及消瘦供体的脂肪间充质干细胞注射到实验性自身免疫性脑脊髓炎小鼠体内,结果显示来自肥胖供体的脂肪间充质干细胞未能抑制炎症反应及临床症状,且肥胖供体的脂肪间充质干细胞使促炎性细胞因子的分泌增加。通过静脉注射移植细胞通常是人们在实验中首选的注射方法,但鼻内给药可绕过血脑屏障,经嗅觉及三叉神经通路直接进入大脑的优势,也为研究者们提供了另一选择[42]。
在DAHBOUR等[43]进行的一项临床试验中,有10例多发性硬化患者接受了骨髓间充质干细胞移植,这10例患者中8例为继发进展型多发性硬化,2例为复发缓解型多发性硬化,在鞘内注射骨髓间充质干细胞1个月后通过同样的方式注射间充质干细胞培养基,并在移植后的第3,6,12个月对患者进行评估。直至随访结束时,这10例患者在评估期间均未出现与治疗相关的严重不良反应;2例继发进展型多发性硬化患者的扩展残疾状况量表评分下降,其余8例患者的扩展残疾状况量表评分较移植前升高或是无明显变化;除病灶体积增加外,其他的检查结果较之前均有改善。在FERNANDEZ等[44]进行的一项三盲Ⅰ/Ⅱ期临床试验中,将34例继发进展型多发性硬化患者随机分配到安慰剂组、低剂量(1×106/kg)脂肪间充质干细胞组、高剂量(4×106/kg)脂肪间充质干细胞组,最终有30例患者参与试验,其中安慰剂组11例,低剂量组10例,高剂量组9例。在12个月的随访期间内,安慰剂组中有2例患者发生死亡,其中1例患者在静脉输注脂肪间充质干细胞2 d因进食后出现窒息而死亡,另外1例患者在移植9个月后因呼吸道感染而死亡;高剂量组出现1例尿路感染;而低剂量组在随访期间内未报告严重不良事件;虽然3组患者的扩展残疾状况量表、MRI评分在随访结束后较移植前均出现改善,但这3组之间的差异却无显著性意义。
虽然在一些临床试验中已经证实了间充质干细胞移植治疗多发性硬化的安全性及有效性,但在移植前选择何种注射途径及注射剂量达到最佳的移植效果仍是今后研究中需要面临的一个挑战。
2.1.3 神经干细胞移植治疗多发性硬化 神经干细胞是一类具有自我更新及分化功能的多能干细胞,成人神经干细胞主要位于侧脑室的脑室下区域和齿状回亚颗粒层区域。有研究表明在小鼠第四脑室表面也能检测到CD133+神经干细胞的存在[13,45]。神经干细胞可以分化为各种神经祖细胞,如少突胶质细胞、神经元和星形胶质细胞,以替代因损伤而丢失的神经细胞[46],包括胚胎或成人脑组织在内的各种细胞类型都可以作为神经干细胞的来源,同时神经干细胞也可以由胚胎干细胞、间充质干细胞和诱导性多能干细胞分化产生[47]。
神经干细胞可能通过以下几种机制治疗多发性硬
化[13,47-48]:①将微小的有害生物转化为神经保护性表型,增加成年小鼠脑中与神经保护表型相关的分子的表达;②发挥其细胞置换、免疫调节、营养支持以及刺激神经祖细胞分化的作用;③作为微环境的传感器,维持体内稳态并优化神经系统。
正如前所述,髓鞘再生是治疗多发性硬化的关键,而这一过程需要神经干细胞的参与,神经干细胞能够迁移到特定的区域并分化为成熟的少突胶质细胞,使受损的神经功能得以恢复[49]。神经干细胞治疗多发性硬化的一个主要问题是病变部位炎症微环境的改变,一些可溶性因子和小胶质细胞均会影响神经干细胞的存活力、分化能力及其向病变部位的迁移能力[2]。目前已有多项研究证实了可以通过不同的体外诱导方法增强神经干细胞移植的效果[50-51]。SUN等[52]研究表明实验性自身免疫性脑脊髓炎小鼠的学习和记忆障碍是由髓磷脂损伤引起的,而MOORE等[51]在之前的研究过程中发现,ERβ选择性激动剂可通过增强实验性自身免疫性脑脊髓炎中的少突胶质细胞分化并改善小鼠中枢神经系统髓鞘再生。但IMAMURA等[50]在最近的研究中表明多奈哌齐可以诱导髓磷脂相关基因的表达,并通过ER信号通路刺激由诱导性多能干细胞衍生的神经干细胞分化为少突胶质细胞,从而进一步改善中枢神经系统内的髓鞘再生。另外,体内的一些代谢产物也会影响神经干细胞移植的效果。体内实验同样证实了移植后的神经干细胞通过SUCNR1信号通路清除琥珀酸酯而表现出抗炎作用。将缺乏SUCNR1的神经干细胞移植到实验性自身免疫性脑脊髓炎小鼠体内,可观察到小胶质细胞未能转变成抗炎表型,并且小鼠的行为能力仅有轻微的恢复[48]。
骨髓来源的神经干细胞和由侧脑室下区衍生的神经干细胞在实验性自身免疫性脑脊髓炎模型中的治疗效果几乎相同,并具有类似的形态学特征,相似的分化为神经元及星形胶质细胞的能力[53]。XIE等[54]将骨髓来源神经干细胞与经转化生长因子β1转染的骨髓来源神经干细胞通过尾静脉分别注射到实验性自身免疫性脑脊髓炎小鼠体内,经转化生长因子β1转染的骨髓来源神经干细胞通过抑制Th1及Th17的表达,促进外周调节性T细胞及白细胞介素10的生成以及将小胶质细胞从M1型转变为M2型,能更有效地抑制小鼠的临床严重程度、中枢神经系统内的炎症反应及脱髓鞘改变。此外,与注射同剂量的生理盐水对照组相比,骨髓来源神经干细胞组及转化生长因子β1转染的骨髓来源神经干细胞组中枢神经系统内神经元及少突胶质细胞的总数明显升高,而神经干细胞治疗的两个组之间却无明显差异,这表明转化生长因子β1不会改变神经干细胞的增殖和分化能力。
在实验性自身免疫性脑脊髓炎小鼠慢性阶段移植人神经干细胞后,小鼠体内的内源性修复途径被激活并伴随着髓鞘再生及中枢神经系统内CD4+CD25+FoxP3+Treg的表达增加;与注射同剂量的磷酸盐缓冲溶液对照组相比,接受人神经干细胞移植治疗的小鼠神经炎症反应明显减轻[55]。而ZHANG等[15]将小鼠诱导性多能干细胞衍生的神经干细胞移植到实验性自身免疫性脑脊髓炎小鼠体内,发现受损的神经组织及髓鞘逐渐恢复,小鼠的运动能力也得到明显提升。
HARRIS等[56]在一项Ⅰ期临床试验中评估了自体骨髓间充质干细胞源性神经祖细胞治疗20例多发性硬化患者(16例为继发进展型多发性硬化,4例为原发进展型多发性硬化)的安全性和耐受性。将自体骨髓间充质干细胞源性神经祖细胞以鞘内注射的方式分3次给药,在每次注射的当天、第2天、1周后、1个月后及第3次注射后的第3个月及第6个月对患者进行随访评估,并在第3次注射的24个月后对这20例患者进行了长期安全性的评估。在完成所有的随访后,这20例患者均未发生与移植治疗相关的严重不良反应,并且在头颅MRI的T2像中未发现新的病灶,这也提示自体骨髓间充质干细胞源性神经祖细胞移植治疗多发性硬化在短期内是安全的。
2.1.4 诱导性多能干细胞移植治疗多发性硬化 诱导性多能干细胞是使用特定转录因子通过重编程从成年体细胞中产生的一类多能干细胞[57]。诱导性多能干细胞具有与胚胎干细胞相似的特性,两者均可以分化为体内所有的细胞类型[2]。除成纤维细胞外,诱导性多能干细胞可由多种体细胞生成,避免了移植后所产生的排斥反应;另外,诱导性多能干细胞的治疗作用不受细胞替代的限制,还具有免疫抑制作用,并为内源性修复机制提供营养支持[58]。这些特点也使诱导性多能干细胞用于多发性硬化的治疗极具吸引力。
由人诱导性多能干细胞衍生的少突胶质祖细胞可以分化为少突胶质细胞,并使中枢神经系统中受损的轴突重新髓鞘化,但这种作用在多发性硬化急性进展期并不明显[59]。将人诱导性多能干细胞衍生的少突胶质前体细胞移植至实验性自身免疫性脑脊髓炎小鼠体内,炎性细胞的浸润明显减少,而在小鼠疾病发作后将小鼠诱导性多能干细胞衍生的神经祖细胞通过鞘内注射的方式移植到小鼠体内也可观察到相似的作用[59]。由诱导性多能干细胞衍生的神经祖细胞既不分化为神经元细胞,也不向少突胶质细胞分化,也没有从血管周围迁移到病变部位,而是通过分泌白血病抑制因子支持小鼠诱导性多能干细胞衍生的神经祖细胞发挥其神经保护作用,白血病抑制因子能够维持少突胶质细胞的存活和分化[59]。ZHANG等[15]研究证实了小鼠诱导性多能干细胞衍生的神经干细胞于脑室内移植可以改善实验性自身免疫性脑脊髓炎小鼠的功能恢复,并抑制T细胞浸润及减轻脑白质的损害。另外,尽管健康的诱导性多能干细胞衍生的神经祖细胞减轻了炎症反应,但源于原发进展型多发性硬化患者的诱导性多能干细胞所衍生的神经祖细胞在脱髓鞘过程中未能提供有益的作
用[60-61],这表明不同物种或给药途径之间的差异对诱导性多能干细胞所产生的作用也有一定的差异。
尽管诱导性多能干细胞具有广泛的临床应用潜力,但有关诱导性多能干细胞治疗多发性硬化仍然存在很多问题:如诱导性多能干细胞初始分化的细胞类型是否适合于多发性硬化的治疗;通过何种途径注射移植的细胞;最后,诱导性多能干细胞的来源及获取也是需要考虑的问题。
2.2 干细胞移植治疗视神经脊髓炎谱系疾病 视神经脊髓炎是一种主要累及脊髓和视神经的脱髓鞘疾病。在过去,人们常把视神经脊髓炎与多发性硬化视作一种疾病,而在之后的研究中人们发现视神经脊髓炎有不同的发病机制及影像学特征,并且具有独特的生物标志物——水通道蛋白4,且视神经脊髓炎的预后相对于多发性硬化较差[7,62]。在2015年关于视神经脊髓炎的诊断标准中引入了视神经脊髓炎谱系疾病这一概念,其中涵盖了所有可能出现的临床表现,从而完善了人们对视神经脊髓炎的认识[63]。视神经脊髓炎谱系疾病的发病率估计为0.05/10万-4.4/10万,且以女性居多[64]。视神经脊髓炎谱系疾病患者的临床症状常表现为视神经炎、急性脊髓炎、区域性视网膜后综合征和脑损伤;若患者未能得到及时有效的治疗,大多数患者会出现上述临床表现的随机复发,而每次临床发作都会恶化视神经脊髓炎谱系疾病患者的神经功能障碍[63,65-66]。目前尚未有研究证明免疫调节剂和抗炎剂会影响视神经脊髓炎的进程,一些用于多发性硬化的有效治疗药物,如干扰素、那他珠单抗等,在视神经脊髓炎患者中都无效,甚至在一些个体上观察到疾病的恶化[67]。因此,缓解视神经脊髓炎谱系疾病患者的症状及预后是目前亟待解决的一个问题。
间充质干细胞对视神经脊髓炎产生的神经保护作用可能是通过调节滤泡辅助性T细胞、白细胞介素6、白细胞介素21的表达,从而阻止该疾病的进展[68]。YANG等[69]研究发现视神经脊髓炎患者来源的骨髓间充质干细胞与健康对照组来源的骨髓间充质干细胞相比,尽管表现出相似的细胞形态和分化能力,但前者的细胞生长速度较慢且更容易衰老,对治疗视神经脊髓炎的效果也会产生影响。SHI等[70]将补体抑制剂-补体因子H相关蛋白1修饰的神经干细胞移植到视神经脊髓炎谱系疾病模型小鼠脑中,发现补体因子H相关蛋白1能够抑制补体激活,阻止膜攻击复合物的形成,保护由神经干细胞分化的星形胶质细胞免受NMO-IgG和补体介导的损伤,阻止疾病的进一步恶化,这表明该移植方法在疾病早期具有治疗作用。
GRECO等[71]评估了2001至2011年期间因免疫抑制疗法无效而接受自体造血干细胞移植治疗的16例难治性视神经脊髓炎患者的效果,在结束历时47个月的中位随访期后,16例患者中有1例患者在移植后的第14个月因疾病进展而死亡,有3例患者的病情未发生进展且无需进行治疗,而其余患者在移植后因残疾进展或是疾病复发需要接受进一步治疗。另外,有病例报告显示在自体造血干细胞移植之前使用利妥昔单抗治疗,可持续缓解视神经脊髓炎患者的临床症状及影像学表现[72]。在新加坡进行的一项临床研究中,对2002至2015年间接受自体造血干细胞移植的3例视神经脊髓炎患者进行4-13年的随访,3例患者的临床症状都较稳定,扩展残疾状况量表评分较移植前均下降且趋于稳定状态,MRI未发现新的病灶[73]。在一项使用非清髓性自体造血干细胞移植治疗视神经脊髓炎的前瞻性研究中[74],共有13例视神经脊髓炎患者接受了此方案的治疗。这13例患者中有12例AQP4-IgG为阳性,含1例合并系统性红斑狼疮患者,且该患者在移植后的第10个月因狼疮并发症而死亡。在自体造血干细胞移植后,9例患者AQP4-IgG转为阴性,并且到最后一次随访时均未出现复发;2例患者在移植后AQP4-IgG仍为阳性,并在移植后2年内出现复发;视神经脊髓炎患者5年内的无进展生存率为90%,无复发生存率为80%;移植前扩展残疾状况量表评分为4.4分,移植后1年为2.8分,而移植后5年为3.3分。因此,缓解自体造血干细胞移植治疗视神经脊髓炎的复发仍是目前面临的一个挑战。
LU等[75]在早些年评估了人脐带间充质干细胞治疗5例视神经脊髓炎谱系疾病患者的效果,在移植后每隔6个月进行1次评估,在经过24个月的随访评估后,5例视神经脊髓炎谱系疾病患者中有4例患者扩展残疾状况量表评分改善,而另1例患者扩展残疾状况量表评分升高;2例患者的视力得到改善;在移植后6个月,患者脊髓MRI上的病灶数明显降低;在整个随访期间内,所有患者均未出现与移植相关的严重不良反应,并且这5例患者的复发率较移植前均下降;另外,免疫学分析表明有4例患者外周血的B细胞含量下降,而T细胞含量增加。FU等[68]近期进行的一项关于间充质干细胞移植治疗视神经脊髓炎谱系疾病的观察性研究中也观察到了类似的效果,视神经脊髓炎谱系疾病患者在间充质干细胞移植后2年的临床复发率有明显的下降,而扩展残疾状况量表评分也从4.9分降低到4.0分。
| [1] TABANSKY I, STERN JNH. Basics of Stem Cell Biology as Applied to the Brain. In: PFAFF D, CHRISTEN Y, editors. Stem Cells in Neuroendocrinology [Internet]. Cham (CH): Springer; 2016. [2] GENC B, BOZAN HR, GENC S, et al. Stem Cell Therapy for Multiple Sclerosis. Adv Exp Med Biol. 2019;1084:145-174. [3] SHROFF G. A review on stem cell therapy for multiple sclerosis: special focus on human embryonic stem cells. Stem Cells Cloning. 2018;11: 1-11. [4] DOUVARAS P, WANG J, ZIMMER M, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3(2):250-259. [5] CHEN B, ZHOU M, OUYANG J, et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci. 2012;33(4):881-886. [6] RICE CM, KEMP K, WILKINS A, et al. Cell therapy for multiple sclerosis: an evolving concept with implications for other neurodegenerative diseases. Lancet. 2013;382(9899):1204-1213. [7] ZHANG P, LIU B. Effect of autologous hematopoietic stem cell transplantation on multiple sclerosis and neuromyelitis optica spectrum disorder: a PRISMA-compliant meta-analysis. Bone Marrow Transplant. 2020;10:1038. [8] LUESSI F, KUHLMANN T, ZIPP F. Remyelinating strategies in multiple sclerosis. Expert Rev Neurother. 2014;14(11):1315-1334. [9] COCLITU C, CONSTANTINESCU CS, TANASESCU R. The future of multiple sclerosis treatments. Expert Rev Neurother. 2016;16(12):1341-1356. [10] MANOUCHEHRINIA A, BEIKI O, HILLERT J. Clinical course of multiple sclerosis: A nationwide cohort study. Mult Scler. 2017;23(11): 1488-1495. [11] OLSSON T, BARCELLOS LF, ALFREDSSON L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25-36. [12] LUESSI F, ZIPP F, WITSCH E. Dendritic cells as therapeutic targets in neuroinflammation. Cell Mol Life Sci. 2016;73(13):2425-2450. [13] XIAO J, YANG R, BISWAS S, et al. Neural Stem Cell-Based Regenerative Approaches for the Treatment of Multiple Sclerosis. Mol Neurobiol. 2018;55(4):3152-3171. [14] ONTANEDA D, THOMPSON AJ, FOX RJ, et al. Progressive multiple sclerosis: prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389(10076):1357-1366. [15] ZHANG C, CAO J, LI X, et al. Treatment of multiple sclerosis by transplantation of neural stem cells derived from induced pluripotent stem cells. Sci China Life Sci. 2016;59(9):950-957. [16] CUASCUT FX, HUTTON GJ. Stem Cell-Based Therapies for Multiple Sclerosis: Current Perspectives. Biomedicines. 2019;7(2):26. [17] BURMAN J, TOLF A, HÄGGLUND H, et al. Autologous haematopoietic stem cell transplantation for neurological diseases. J Neurol Neurosurg Psychiatry. 2018;89(2):147-155. [18] LOH YS, HWANG WY, RATNAGOPAL P. Autologous haematopoietic stem cell transplantation for the treatment of multiple sclerosis. Ann Acad Med Singapore. 2007;36(6):421-426. [19] ARRUDA LCM, DE AZEVEDO JTC, DE OLIVEIRA GLV, et al. Immunological correlates of favorable long-term clinical outcome in multiple sclerosis patients after autologous hematopoietic stem cell transplantation. Clin Immunol. 2016;169:47-57. [20] ZEHER M, PAPP G, NAKKEN B, et al. Hematopoietic stem cell transplantation in autoimmune disorders: From immune-regulatory processes to clinical implications. Autoimmun Rev. 2017;16(8):817-825. [21] KARNELL FG, LIN D, MOTLEY S, et al. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin Exp Immunol. 2017;189(3):268-278. [22] BURMAN J, FRANSSON M, TÖTTERMAN TH, et al. T-cell responses after haematopoietic stem cell transplantation for aggressive relapsing-remitting multiple sclerosis. Immunology. 2013;140(2):211-219. [23] DE PAULA A SOUSA A, MALMEGRIM KC, PANEPUCCI RA, et al. Autologous haematopoietic stem cell transplantation reduces abnormalities in the expression of immune genes in multiple sclerosis. Clin Sci (Lond). 2015;128(2):111-120. [24] RADAELLI M, MERLINI A, GRECO R, et al. Autologous bone marrow transplantation for the treatment of multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14(9):478. [25] NASH RA, HUTTON GJ, RACKE MK, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88(9):842-852. [26] DAS J, SHARRACK B, SNOWDEN JA. Autologous Haematopoietic Stem Cell Transplantation in Multiple Sclerosis: a Review of Current Literature and Future Directions for Transplant Haematologists and Oncologists. Curr Hematol Malig Rep. 2019;14(2):127-135. [27] SHARRACK B, SACCARDI R, ALEXANDER T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2020;55(2):283-306. [28] MURARO PA, MARTIN R, MANCARDI GL, et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13(7):391-405. [29] ARDESHIRY LAJIMI A, HAGH MF, SAKI N, et al. Feasibility of cell therapy in multiple sclerosis: a systematic review of 83 studies. Int J Hematol Oncol Stem Cell Res. 2013;7(1):15-33. [30] BURT RK, BALABANOV R, BURMAN J, et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA. 2019; 321(2):165-174. [31] TOLF A, FAGIUS J, CARLSON K, et al. Sustained remission in multiple sclerosis after hematopoietic stem cell transplantation. Acta Neurol Scand. 2019;140(5):320-327. [32] BALDASSARI LE, COHEN JA. Mesenchymal Stem Cell-derived Neural Progenitor Cells in Progressive Multiple Sclerosis: Great Expectations. EBioMedicine. 2018;29:5-6. [33] HEATHMAN TRJ, RAFIQ QA, CHAN AKC, et al. Characterization of human mesenchymal stem cells from multiple donors and the implications for large scale bioprocess development. Biochem Eng J. 2016;108:14-23. [34] SARKAR P, RICE CM, SCOLDING NJ. Cell Therapy for Multiple Sclerosis. CNS Drugs. 2017;31(6):453-469. [35] BARATI S, RAGERDI KASHANI I, MORADI F, et al. Mesenchymal stem cell mediated effects on microglial phenotype in cuprizone-induced demyelination model. J Cell Biochem. 2019;120(8):13952-13964. [36] SARGENT A, BAI L, SHANO G, et al. CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells. Exp Neurol. 2017;295:222-232. [37] WANG X, KIMBREL EA, IJICHI K, et al. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Reports. 2014;3(1):115-130. [38] SHALABY SM, SABBAH NA, SABER T, et al. Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model. IUBMB Life. 2016;68(2):106-115. [39] LI J, CHEN Y, CHEN Z, et al. Therapeutic effects of human adipose tissue-derived stem cell (hADSC) transplantation on experimental autoimmune encephalomyelitis (EAE) mice. Sci Rep. 2017;7:42695. [40] KURTE M, BRAVO-ALEGRÍA J, TORRES A, et al. Intravenous administration of bone marrow-derived mesenchymal stem cells induces a switch from classical to atypical symptoms in experimental autoimmune encephalomyelitis. Stem Cells Int. 2015;2015:140170. [41] STRONG AL, BOWLES AC, WISE RM, et al. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Stem Cells. 2016;34(3):614-626. [42] LOCHHEAD JJ, THORNE RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614-628. [43] DAHBOUR S, JAMALI F, ALHATTAB D, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. Version 2. CNS Neurosci Ther. 2017;23(11):866-874. [44] FERNÁNDEZ O, IZQUIERDO G, FERNÁNDEZ V, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One. 2018; 13(5):e0195891. [45] LUO Y, COSKUN V, LIANG A, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161(5): 1175-1186. [46] GONZALEZ R, HAMBLIN MH, LEE JP. Neural Stem Cell Transplantation and CNS Diseases. CNS Neurol Disord Drug Targets. 2016;15(8): 881-886. [47] VOLPE G, BERNSTOCK JD, PERUZZOTTI-JAMETTI L, et al. Modulation of host immune responses following non-hematopoietic stem cell transplantation: Translational implications in progressive multiple sclerosis. J Neuroimmunol. 2019;331:11-27. [48] KOKAIA Z, LINDVALL O. Sensors of Succinate: Neural Stem Cell Grafts Fight Neuroinflammation. Cell Stem Cell. 2018;22(3):283-285. [49] KLOSE J, SCHMIDT NO, MELMS A, et al. Suppression of experimental autoimmune encephalomyelitis by interleukin-10 transduced neural stem/progenitor cells. J Neuroinflammation. 2013;10:117. [50] IMAMURA O, ARAI M, DATEKI M, et al. Donepezil-induced oligodendrocyte differentiation is mediated through estrogen receptors. J Neurochem. 2019:e14927. [51] MOORE SM, KHALAJ AJ, KUMAR S, et al. Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. 2014;111(50): 18061-18066. [52] SUN JJ, REN QG, XU L, et al. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Sci Rep. 2015;5:14235. [53] YANG J, YAN Y, CIRIC B, et al. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177(4):1989-2001. [54] XIE C, LI X, ZHOU X, et al. TGFβ1 transduction enhances immunomodulatory capacity of neural stem cells in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2018;69:283-295. [55] MCINTYRE LL, GREILACH SA, OTHY S, et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol Dis. 2020;140:104868. [56] HARRIS VK, STARK J, VYSHKINA T, et al. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine. 2018;29:23-30. [57] HARDING J, MIROCHNITCHENKO O. Preclinical studies for induced pluripotent stem cell-based therapeutics. J Biol Chem. 2014;289(8): 4585-4593. [58] XIE C, LIU YQ, GUAN YT, et al. Induced Stem Cells as a Novel Multiple Sclerosis Therapy. Curr Stem Cell Res Ther. 2016;11(4):313-320. [59] LATERZA C, MERLINI A, DE FEO D, et al. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun. 2013;4:2597. [60] THIRUVALLUVAN A, CZEPIEL M, KAP YA, et al. Survival and Functionality of Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes in a Nonhuman Primate Model for Multiple Sclerosis. Stem Cells Transl Med. 2016;5(11):1550-1561. [61] NICAISE AM, BANDA E, GUZZO RM, et al. iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Exp Neurol. 2017;288:114-121. [62] WEINSHENKER BG, WINGERCHUK DM. Neuromyelitis Spectrum Disorders. Mayo Clin Proc. 2017;92(4):663-679. [63] WINGERCHUK DM, BANWELL B, BENNETT JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [64] PITTOCK SJ, LUCCHINETTI CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci. 2016;1366(1):20-39. [65] AKAISHI T, NAKASHIMA I, TAKAHASHI T, et al. Neuromyelitis optica spectrum disorders with unevenly clustered attack occurrence. Neurol Neuroimmunol Neuroinflamm. 2019;7(1):e640. [66] KESSLER RA, MEALY MA, LEVY M. Treatment of Neuromyelitis Optica Spectrum Disorder: Acute, Preventive, and Symptomatic. Curr Treat Options Neurol. 2016;18(1):2. [67] KLEITER I, HELLWIG K, BERTHELE A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69(2): 239-245. [68] FU Y, YAN Y, QI Y, et al. Impact of Autologous Mesenchymal Stem Cell Infusion on Neuromyelitis Optica Spectrum Disorder: A Pilot, 2-Year Observational Study. CNS Neurosci Ther. 2016;22(8):677-685. [69] YANG C, YANG Y, MA L, et al. Study of the cytological features of bone marrow mesenchymal stem cells from patients with neuromyelitis optica. Int J Mol Med. 2019;43(3):1395-1405. [70] SHI K, WANG Z, LIU Y, et al. CFHR1-Modified Neural Stem Cells Ameliorated Brain Injury in a Mouse Model of Neuromyelitis Optica Spectrum Disorders. J Immunol. 2016;197(9):3471-3480. [71] GRECO R, BONDANZA A, OLIVEIRA MC, et al. Autologous hematopoietic stem cell transplantation in neuromyelitis optica: a registry study of the EBMT Autoimmune Diseases Working Party. Mult Scler. 2015;21(2): 189-197. [72] AOUAD P, LI J, ARTHUR C, et al. Resolution of aquaporin-4 antibodies in a woman with neuromyelitis optica treated with human autologous stem cell transplant. J Clin Neurosci. 2015;22(7):1215-1217. [73] HOAY KY, RATNAGOPAL P. Autologous Hematopoietic Stem Cell Transplantation for the Treatment of Neuromyelitis Optica in Singapore. Acta Neurol Taiwan. 2018;27(1):26-32. [74] BURT RK, BALABANOV R, HAN X, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology. 2019;93(18):e1732-e1741. [75] LU Z, YE D, QIAN L, et al. Human umbilical cord mesenchymal stem cell therapy on neuromyelitis optica. Curr Neurovasc Res. 2012;9(4): 250-255. |
| [1] | 林清凡, 解一新, 陈婉清, 叶振忠, 陈幼芳. 人胎盘源间充质干细胞条件培养液可上调缺氧状态下BeWo细胞活力和紧密连接因子的表达[J]. 中国组织工程研究, 2021, 25(在线): 4970-4975. |
| [2] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [3] | 张 超, 吕 欣. 髋臼骨折固定后的异位骨化:危险因素、预防及其治疗进展[J]. 中国组织工程研究, 2021, 25(9): 1434-1439. |
| [4] | 周继辉, 李新志, 周 游, 黄 卫, 陈文瑶. 髌骨骨折修复内植物选择的多重问题[J]. 中国组织工程研究, 2021, 25(9): 1440-1445. |
| [5] | 王德斌, 毕郑刚. 尺骨鹰嘴骨折-脱位解剖力学、损伤特点、固定修复及3D技术应用的相关问题[J]. 中国组织工程研究, 2021, 25(9): 1446-1451. |
| [6] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [7] | 姬志祥, 蓝常贡. 尿酸盐转运蛋白在痛风中的多态性和治疗相关性[J]. 中国组织工程研究, 2021, 25(8): 1290-1298. |
| [8] | 袁 美, 张新新, 郭祎莎, 毕 霞. 循环microRNA在血管性认知障碍诊断中的应用[J]. 中国组织工程研究, 2021, 25(8): 1299-1304. |
| [9] | 王正东, 黄 娜, 陈婧娴, 郑作兵, 胡鑫宇, 李 梅, 苏 晓, 苏学森, 颜 南. 丁酸钠抑制氟中毒可诱导小胶质细胞活化及炎症因子表达增多[J]. 中国组织工程研究, 2021, 25(7): 1075-1080. |
| [10] | 汪显耀, 关亚琳, 刘忠山. 提高间充质干细胞治疗难愈性创面的策略[J]. 中国组织工程研究, 2021, 25(7): 1081-1087. |
| [11] | 万 然, 史 旭, 刘京松, 王岩松. 间充质干细胞分泌组治疗脊髓损伤的研究进展[J]. 中国组织工程研究, 2021, 25(7): 1088-1095. |
| [12] | 廖成成, 安家兴, 谭张雪, 王 倩, 刘建国. 口腔鳞状细胞癌干细胞的治疗靶点及应用前景[J]. 中国组织工程研究, 2021, 25(7): 1096-1103. |
| [13] | 赵 敏, 冯柳祥, 陈 垚, 顾 霞, 王平义, 李一梅, 李文华. 低氧环境下外泌体可作为疾病的标志物[J]. 中国组织工程研究, 2021, 25(7): 1104-1108. |
| [14] | 谢文佳, 夏天娇, 周卿云, 刘羽佳, 顾小萍. 小胶质细胞介导神经元损伤在神经退行性疾病中的作用[J]. 中国组织工程研究, 2021, 25(7): 1109-1115. |
| [15] | 李珊珊, 郭笑霄, 尤 冉, 杨秀芬, 赵 露, 陈 曦, 王艳玲. 感光细胞替代治疗视网膜变性疾病[J]. 中国组织工程研究, 2021, 25(7): 1116-1121. |
干细胞是一类未分化的细胞,具有自我更新能力,可通过不对称分裂分化为某种特定组织类型的细胞[1]。干细胞根据来源可分为2种类型:胚胎干细胞和成体干细胞。胚胎干细胞可以分化为除胚外组织以外的所有组织类型,而成体干细胞具有产生组织特异性细胞类型的能力[2]。由于干细胞可替代受损或丢失的神经组织,提供免疫抑制、修复和神经保护作用,使干细胞在神经系统相关疾病中的应用也越来越广泛[3]。对于干细胞治疗神经系统相关疾病的机制,学者们提出了以下3种假设:①干细胞替代了中枢神经系统中受损的少突胶质细胞;②通过造血干细胞重建机体的免疫系统,以恢复患者的免疫能力;③利用内源性干细胞修复或保护神经组织[4-6]。相关研究表明,与目前常用的免疫抑制疗法相比,干细胞移植治疗可长期缓解多发性硬化及视神经脊髓炎谱系疾病患者的临床症状,改善患者的预后[7]。文章就不同干细胞在多发性硬化及视神经脊髓炎谱系疾病中的作用做一综述。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.2 文献选择
选入标准:干细胞治疗多发性硬化、视神经脊髓炎谱系疾病的相关文献。
排除标准:①重复文献;②会议类、评论类、摘要类文献;③与研究目的不符的相关文献。
1.3 资料提取与文献质量评价 通过关键词对文献进行初筛,根据排除标准以及在阅读文献摘要后删除相关文献,最终对75篇文献进行综述分析。检索流程见图1。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程

文题释义:#br# 脱髓鞘疾病:髓鞘是包裹在神经细胞轴突外面的一层膜,其绝缘作用可以防止神经电冲动从神经元轴突传递至另一神经元轴突。脱髓鞘是指髓鞘形成后发生的髓鞘损坏,脱髓鞘疾病是以神经髓鞘脱失为主,神经元胞体及轴突相对受累较轻为特征的一组疾病。多发性硬化及视神经脊髓炎谱系疾病都属于中枢神经系统脱髓鞘疾病。#br# 干细胞治疗中枢神经系统脱髓鞘疾病的价值:近年来,干细胞在一些自身免疫性疾病中的应用越来越广泛。由于干细胞对受损的神经组织、轴突等具有保护及修复作用,使其在治疗诸如多发性硬化、视神经脊髓炎谱系疾病等脱髓鞘疾病中的应用受到大家的青睐。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||