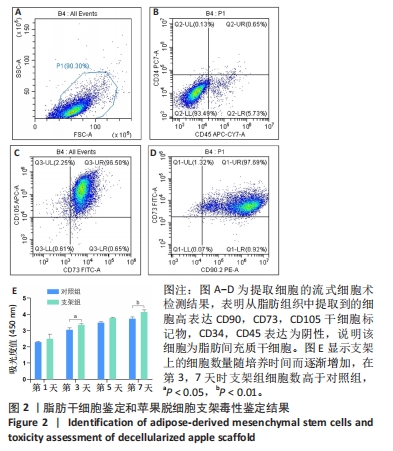
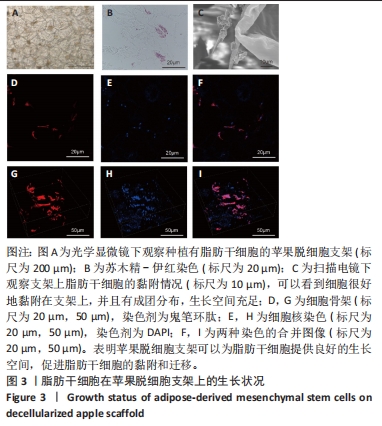
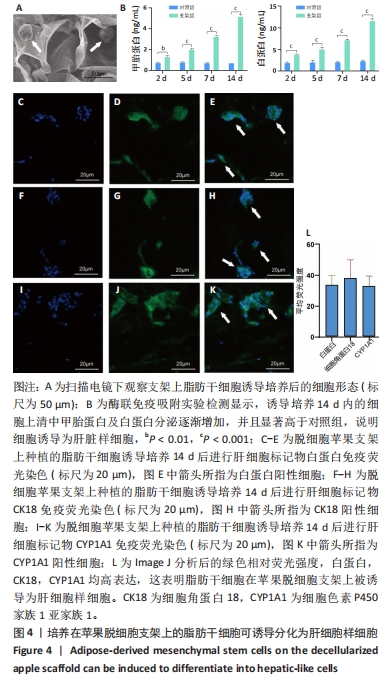
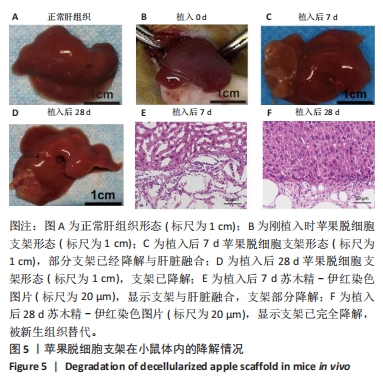
[1] WANG FS, FAN JG, ZHANG Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099-2108.
[2] XIAO J, WANG F, WONG NK, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019; 71(1):212-221.
[3] TUJIOS S, STRAVITZ RT, LEE WM. Management of acute liver failure: update 2022. Semin Liver Dis. 2022;42(3):362-378.
[4] AGARWAL B, CANIZARES RB, SALIBA F, et al. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure. J Hepatol. 2023; 79(1):79-92.
[5] SALIBA F, BANARES R, LAESEN FS, et al. Artificial liver support in patients with liver failure: a modified DELPHI consensus of international experts. Intensive Care Med. 2022;48(10):1352-1367.
[6] WANG Y, ZHENG Q, SUN Z, et al. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes. Cell Stem Cell. 2023;30(5):617-631.
[7] XU W, ZHU S, YANG L, et al. Safety and efficacy of double plasma molecular adsorption system with sequential low-volume plasma exchange in intermediate-stage hepatitis B virus-related acute-on-chronic liver failure. J Med Virol. 2023;95(3):e28650.
[8] GIGLIO MC, DOLCE P, YILMAZ S, et al. European Liver and Intestine Transplant Association (ELITA). Development of a model to predict the risk of early graft failure after adult-to-adult living donor liver transplantation: an ELTR study. Liver Transpl. 2023. doi: 10.1097/LVT.0000000000000312.
[9] SARIN SK, CHOUDHURY A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016; 13(3):131-149.
[10] LUO J, LI J, LI P, et al. Acute-on-chronic liver failure: far to go-a review. Crit Care. 2023;27(1):259.
[11] HONG G, KIM J, OH H, et al. Production of multiple cell-laden microtissue spheroids with a biomimetic hepatic-lobule-like structure. Adv Mater. 2021;33(36):e2102624.
[12] BERNAL PN, BOUWMEESTER M, MADRID-WOLFF J, et al. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv Mater. 2022;34(15):e2110054.
[13] TAYMOUR R, CHICAIZA-CABEZAS NA, GELINSKY M, et al. Core-shell bioprinting of vascularizedin vitroliver sinusoid models. Biofabrication. 2022. doi: 10.1088/1758-5090/ac9019.
[14] HEYDARI Z, NAJIMI M, MIRZAEI H, et al. Tissue engineering in liver regenerative medicine: insights into novel translational technologies. Cells. 2020;9(2):304.
[15] XU L, WANG S, SUI X, et al. Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure. ACS Appl Mater Interfaces. 2017;9(17): 14716-14723.
[16] FURLANI D, UGURLUCAN M, ONG L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77(3):370-376.
[17] BHATIA SN, UNDERHILL GH, ZARET KS, et al. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6(245):245sr2.
[18] JIAN H, LI X, DONG Q, et al. In vitro construction of liver organoids with biomimetic lobule structure by a multicellular 3D bioprinting strategy. Cell Prolif. 2023;56(5):e13465.
[19] HOSSEINI V, MAROUFI NF, SAGHATI S, et al. Current progress in hepatic tissue regeneration by tissue engineering. J Transl Med. 2019; 17(1):383.
[20] JIANKANG H, DICHEN L, YAXIONG L, et al. Preparation of chitosan-gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009;5(1):453-461.
[21] GRIGORYAN B, PAULSEN SJ, CORBETT DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458-464.
[22] CHANDRA P, ATALA A. Engineering blood vessels and vascularized tissues: technology trends and potential clinical applications. Clin Sci (Lond). 2019;133(9):1115-1135.
[23] WANG L, WANG C, WANG Z, et al. Transforming the spleen into a liver-like organ in vivo. Sci Adv. 2020;6(24):eaaz9974.
[24] YANG W, CHEN Q, XIA R, et al. A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 2018;177:52-66.
[25] WU Q, LI Y, YANG Z, et al. Ectopic expansion and vascularization of engineered hepatic tissue based on heparinized acellular liver matrix and mesenchymal stromal cell spheroids. Acta Biomater. 2022;137:79-91.
[26] LE GUILCHER C, MERLEN G, DELLAQUILA A, et al. Engineered human liver based on pullulan-dextran hydrogel promotes mice survival after liver failure. Mater Today Bio. 2023;19:100554.
[27] LEE JW, CHOI YJ, YONG WJ, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1):015007.
[28] KASTURI M, MATHUR V, GADRE M, et al. Three dimensional bioprinting for hepatic tissue engineering: from in vitro models to clinical applications. Tissue Eng Regen Med. 2023; 21(1):21-52.
[29] ZHENG Y, CHEN J, CRAVEN M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109: 9342-9347.
[30] ZHENG X, SHEN G, WANG C, et al. Bio-inspired Murray materials for mass transfer and activity. Nat Commun. 2017;8:14921.
[31] GERSHLAK JR, HERNANDEZ S, FONTANA G, et al. Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials. 2017;125:13-22.
[32] CONTESSI NEGRINI N, TOFFOLETTO N, FARÈ S, et al. Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front Bioeng Biotechnol. 2020;8:723.
[33] CHENG YW, SHIWARSKI DJ, BALL RL, et al. Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomater Sci Eng. 2020;6(5):3046-3054.
[34] HICKEY RJ, MODULEVSKY DJ, CUERRIER CM, et al. Customizing the shape and microenvironment biochemistry of biocompatible macroscopic plant-derived cellulose scaffolds. ACS Biomater Sci Eng. 2018;4(11):3726-3736.
[35] BAI H, XIE B, WANG Z, et al. Application of the tissue-engineered plant scaffold as a vascular patch. ACS Omega. 2021;6(17):11595-11601.
[36] FONTANA G, GERSHLAK J, ADAMSKI M, et al. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv Healthc Mater. 2017. doi: 10.1002/adhm.201601225.
[37] MODULEVSKY DJ, CUERRIER CM, PELLING AE. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS One. 2016;11(6):e0157894.
[38] S H A, MOHAN CC, P S U, et al. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater Sci Eng C Mater Biol Appl. 2021;119:111500.
[39] HICKEY RJ, PELLING AE. Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol. 2019;7:45.
[40] SONG Z, HAN H, GE X, et al. Deficiency of neutrophil high-mobility group box-1 in liver transplant recipients exacerbates early allograft injury in mice. Hepatology. 2023;78(3):771-786.
[41] KULKARNI AV, REDDY R, SHARMA M, et al. Healthcare utilization and outcomes of living donor liver transplantation for patients with APASL-defined acute-on-chronic liver failure. Hepatol Int. 2023;17(5): 1233-1240.
[42] SCHLEGEL A, VAN REEVEN M, CROOME K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2022;76(2):371-382.
[43] KIM W, GWON Y, PARK S, et al. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2022;19:50-74.
[44] LIU C, WANG L, XU M, et al. Reprogramming the spleen into a functioning ‘liver’ in vivo. Gut. 2022;71(11):2325-2336.
[45] VELAZQUEZ JJ, LEGRAW R, MOGHADAM F, et al. Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 2021; 12(1):41-55.e11.
[46] SPHABMIXAY P, RAREDON MSB, WANG AJ, et al. High resolution stereolithography fabrication of perfusable scaffolds to enable long-term meso-scale hepatic culture for disease modeling. Biofabrication. 2021. doi: 10.1088/1758-5090/ac23aa.
[47] PROVIN C, TAKANO K, YOSHIDA T, et al. Low O2 metabolism of HepG2 cells cultured at high density in a 3D microstructured scaffold. Biomed Microdevices. 2009;11(2):485-494.
[48] GAO Y, CALLANAN A. Influence of surface topography on PCL electrospun scaffolds for liver tissue engineering. J Mater Chem B. 2021;9(38):8081-8093.
[49] HE J, ZHOU C, XU X, et al. Scalable formation of highly viable and functional hepatocellular carcinoma spheroids in an oxygen-permeable microwell device for anti-tumor drug evaluation. Adv Healthc Mater. 2022;11(18):e2200863.
[50] MA P, JIANG L, LUO X, et al. Hybrid polydimethylsiloxane (PDMS) incorporated thermogelling system for effective liver cancer treatment. Pharmaceutics. 2022;14(12):2623.
[51] BADEKILA AK, PAI V, VIJAYAN V, et al. Engineering alginate/carboxymethylcellulose scaffolds to establish liver cancer spheroids: Evaluation of molecular variances between 2D and 3D models. Int J Biol Macromol. 2023;254(Pt 3):128058.
[52] KHODABAKHSH AGHDAM S, KHOSHFETRAT AB, RAHBARGHAZI R, et al. Collagen modulates functional activity of hepatic cells inside alginate-galactosylated chitosan hydrogel microcapsules. Int J Biol Macromol. 2020;156:1270-1278.
[53] SHIT A, PARK S, LEE Y, et al. Stimuli-responsive pressure-strain sensor-based conductive hydrogel for alleviated non-alcoholic fatty liver disease by scavenging reactive oxygen species in adipose tissue. Acta Biomater. 2023;171:406-416.
[54] VASUDEVAN A, MAJUMDER N, SHARMA I, et al. Liver extracellular matrix-based nanofiber scaffolds for the culture of primary hepatocytes and drug screening. ACS Biomater Sci Eng. 2023;9(11):6357-6368.
[55] SONG Y, LIU C, XU X, et al. Chitosan-based multifunctional hydrogel with bio-adhesion and antioxidant properties for efficient wound hemostasis. Colloids Surf B Biointerfaces. 2023;234:113697.
[56] CHEN Z, CHEN L, KHOO KS, et al. Exploitation of lignocellulosic-based biomass biorefinery: a critical review of renewable bioresource, sustainability and economic views. Biotechnol Adv. 2023;69:108265.
[57] HACHIMI ALAOUI C, RÉTHORÉ G, WEISS P, et al. Sustainable biomass lignin-based hydrogels: a review on properties, formulation, and biomedical applications. Int J Mol Sci. 2023;24(17):13493.
[58] ISWARYA S, THEIVASANTHI T, GOPINATH SCB. Sodium alginate/hydroxyapatite/nanocellulose composites: synthesis and potentials for bone tissue engineering. J Mech Behav Biomed Mater. 2023;148: 106189.
[59] YE J, LI J, WANG X, et al. Preparation of bacterial cellulose-based antibacterial membranes with prolonged release of drugs: emphasis on the chemical structure of drugs. Carbohydr Polym. 2024;323:121379.
[60] ZHENG Z, ZHANG H, QIAN K, et al. Wood structure-inspired injectable lignin-based nanogels as blood-vessel-embolic sustained drug-releasing stent for interventional therapies on liver cancer. Biomaterials. 2023; 302:122324.
|