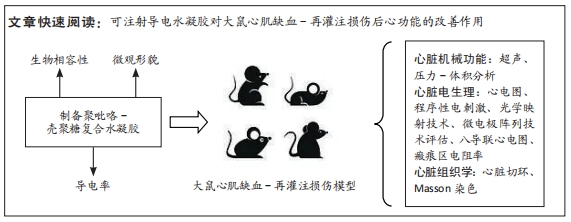
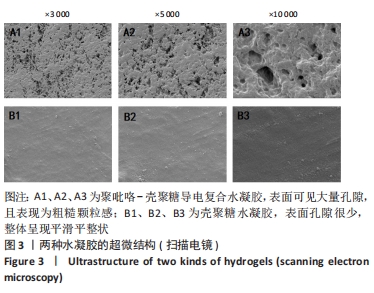
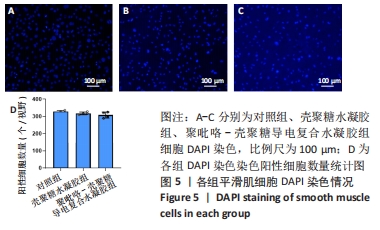
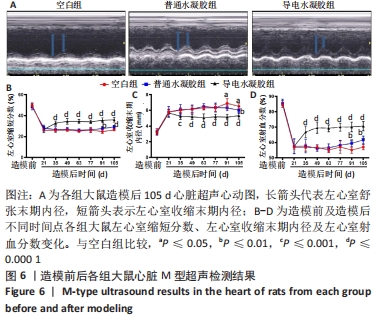
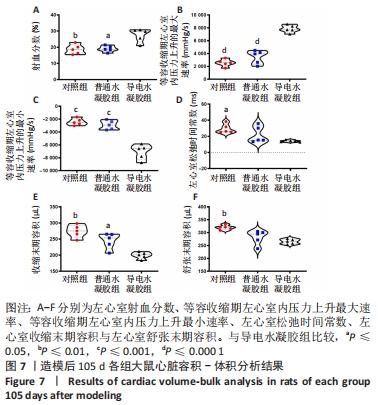
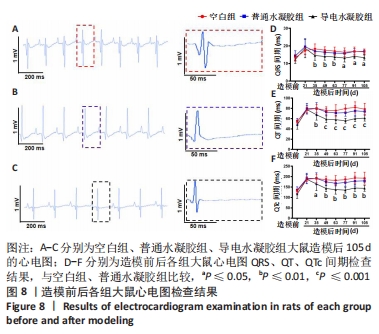
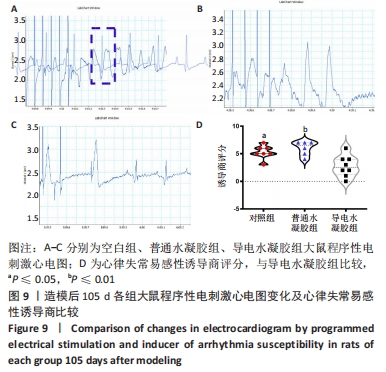
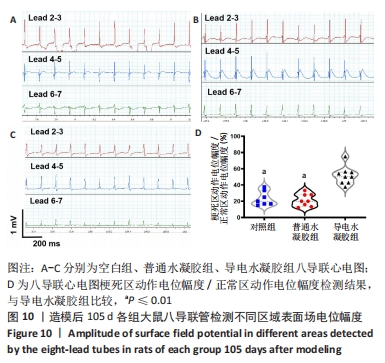
[1] HERR DJ, SINGH T, DHAMMU T, et al. Regulation of metabolism by mitochondrial enzyme acetylation in cardiac ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165728.
[2] MARINESCU MC, LAZAR AL, MARTA MM, et al. Non-Coding RNAs: Prevention, Diagnosis, and Treatment in Myocardial Ischemia-Reperfusion Injury. Int J Mol Sci. 2022;23(5):2728.
[3] BRISTOW MR, SAXON LA, BOEHMER J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140-2150.
[4] CLELAND JG, DAUBERT JC, ERDMANN E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539-1549.
[5] HE S, WU J, LI SH, et al. The conductive function of biopolymer corrects myocardial scar conduction blockage and resynchronizes contraction to prevent heart failure. Biomaterials. 2020;258:120285.
[6] GABETTI S, SILEO A, MONTRONE F, et al. Versatile electrical stimulator for cardiac tissue engineering-Investigation of charge-balanced monophasic and biphasic electrical stimulations. Front Bioeng Biotechnol. 2022;10:1031183.
[7] CUI Z, NI NC, WU J, et al. Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics. 2018;8(10):2752-2764.
[8] MASSOUMI B, ABBASIAN M, JAHANBAN-ESFAHLAN R, et al. A novel bio-inspired conductive, biocompatible, and adhesive terpolymer based on polyaniline, polydopamine, and polylactide as scaffolding biomaterial for tissue engineering application. Int J Biol Macromol. 2020;147:1174-1184.
[9] LEE M, KIM MC, LEE JY. Nanomaterial-Based Electrically Conductive Hydrogels for Cardiac Tissue Repair. Int J Nanomedicine. 2022;17: 6181-6200.
[10] WANJARE M, KAWAMURA M, HU C, et al. Vascularization of Engineered Spatially Patterned Myocardial Tissue Derived From Human Pluripotent Stem Cells in vivo. Front Bioeng Biotechnol. 2019;7:208.
[11] BEAUCHAMP P, JACKSON CB, OZHATHIL LC, et al. 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front Mol Biosci. 2020;7:14.
[12] LI Y, QIU X. Bioelectricity-coupling patches for repairing impaired myocardium. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(4):e1787.
[13] LI Y, WEI L, LAN L, et al. Conductive biomaterials for cardiac repair: A review. Acta Biomater. 2022;139:157-178.
[14] QUE W, HAN C, ZHAO X, et al. An ECG generative model of myocardial infarction. Comput Methods Programs Biomed. 2022;225:107062.
[15] FAVERE K, VAN FRAEYENHOVE J, JACOBS G, et al. Cardiac electrophysiology studies in mice via the transjugular route: a comprehensive practical guide. Am J Physiol Heart Circ Physiol. 2022;323(4):H763-h773.
[16] 姜增誉,阎长平,李健丁,等.新型导电复合材料聚吡咯壳聚糖对新生大鼠心室肌细胞钙信号传导的影响及可能机制[J].中西医结合心脑血管病杂志,2018,16(12): 1650-1655.
[17] KOLETSI D, ILIADI A, TZANETAKIS GN, et al. Cardiovascular Disease and Chronic Endodontic Infection. Is There an Association? A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18(17):9111.
[18] WANG Y, LI G, YANG L, et al. Development of Innovative Biomaterials and Devices for the Treatment of Cardiovascular Diseases. Adv Mater. 2022;34(46):e2201971.
[19] HE M, WANG D, XU Y, et al. Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease. Pharmaceutics. 2022;14(7):1345.
[20] AGGARWAL M, AGGARWAL B, RAO J. Integrative Medicine for Cardiovascular Disease and Prevention. Med Clin North Am. 2017;101(5):895-923.
[21] DE GREGORIO C. Physical Training and Cardiac Rehabilitation in Heart Failure Patients. Adv Exp Med Biol. 2018;1067:161-181.
[22] 郎丽敏,何生,姜增誉,等.导电复合材料在心肌梗死组织工程治疗领域的应用进展[J].中国组织工程研究,2021,25(22):3584-3590.
[23] MCCARTNEY SL, PATEL C, DEL RIO JM. Long-term outcomes and management of the heart transplant recipient. Best Pract Res Clin Anaesthesiol. 2017;31(2):237-248.
[24] LI Y, WEI L, LAN L, et al. Conductive biomaterials for cardiac repair: A review. Acta Biomater. 2022;139:157-178.
[25] CHO S, DISCHER DE, LEONG KW, et al. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat Methods. 2022;19(9):1064-1071.
[26] MADONNA R, VAN LAAKE LW, BOTKER HE, et al. ESC Working Group on Cellular Biology of the Heart: position paper for Cardiovascular Research: tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc Res. 2019;115(3):488-500.
[27] ASHTARI K, NAZARI H, KO H, et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv Drug Deliv Rev. 2019;144:162-179.
[28] PHUTANE P, TELANGE D, AGRAWAL S, et al. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers (Basel). 2023;15(5):1202.
[29] GHOVVATI M, KHARAZIHA M, ARDEHALI R, et al. Recent Advances in Designing Electroconductive Biomaterials for Cardiac Tissue Engineering. Adv Healthc Mater. 2022; 11(13):e2200055.
[30] MOND HG, HELLAND JR, STOKES K, et al. The electrode-tissue interface: the revolutionary role of steroid-elution. Pacing Clin Electrophysiol. 2014;37(9):1232-1249.
[31] BLACKBURN NJ, SOFRENOVIC T, KURAITIS D, et al. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials. 2015;39:182-192.
[32] CUPA J, STREBEL I, BADERTSCHER P, et al. Diagnostic and prognostic value of QRS duration and QTc interval in patients with suspected myocardial infarction. Cardiol J. 2018;25(5):601-610.
[33] JASTRZĘBSKI M, MOSKAL P, HUYBRECHTS W, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm. 2022;19(1):13-21.
[34] THEUNS D, VAN BOVEN N, SCHAER BA, et al. Predicting Early Mortality Among Implantable Defibrillator Patients Treated With Cardiac Resynchronization Therapy. J Card Fail. 2019; 25(10):812-818.
[35] BEHAR JM, JACKSON T, HYDE E, et al. Optimized Left Ventricular Endocardial Stimulation Is Superior to Optimized Epicardial Stimulation in Ischemic Patients With Poor Response to Cardiac Resynchronization Therapy: A Combined Magnetic Resonance Imaging, Electroanatomic Contact Mapping, and Hemodynamic Study to Target Endocardial Lead Placement. JACC Clin Electrophysiol. 2016;2(7):799-809.
[36] MORSINK M, SEVERINO P, LUNA-CERON E, et al. Effects of electrically conductive nano-biomaterials on regulating cardiomyocyte behavior for cardiac repair and regeneration. Acta Biomater. 2022;139:141-156.
[37] MOUSAVI A, VAHDAT S, BAHEIRAEI N, et al. Multifunctional Conductive Biomaterials as Promising Platforms for Cardiac Tissue Engineering. ACS Biomater Sci Eng. 2021;7(1):55-82.
[38] UL HAQ A, CAROTENUTO F, DE MATTEIS F, et al. Intrinsically Conductive Polymers for Striated Cardiac Muscle Repair. Int J Mol Sci. 2021;22(16):8550.
[39] UL HAQ A, CAROTENUTO F, DI NARDO P, et al. Extrinsically Conductive Nanomaterials for Cardiac Tissue Engineering Applications. Micromachines (Basel). 2021;12(8):914.
[40] WANG L, LIU Y, YE G, et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat Biomed Eng. 2021;5(10):1157-1173.
|