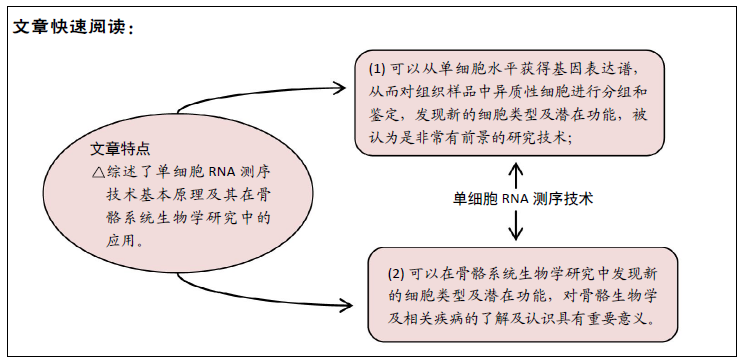
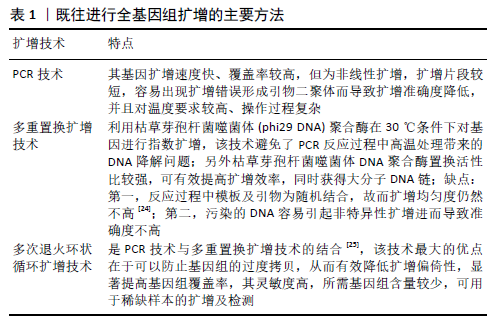
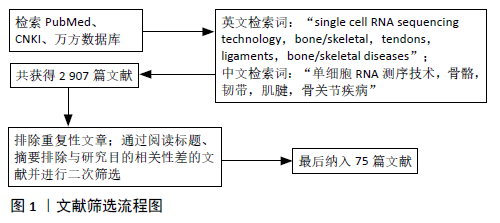
[1] OUYANG HW, CAO T, ZOU XH, et al. Mesenchymal stem cell sheets revitalize nonviable dense grafts: implications for repair of large-bone and tendon defects. Transplantation. 2006;82(2):170-174.
[2] OUYANG HW, GOH JC, LEE EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32(2):321-327.
[3] OUYANG HW, GOH JC, MO XM, et al. The efficacy of bone marrow stromal cell-seeded knitted PLGA fiber scaffold for Achilles tendon repair. Ann N Y Acad Sci. 2002;961:126-129.
[4] ALLISON DC, CARNEY SC, AHLMANN ER, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012; 2012:704872.
[5] KANSARA M, TSANG M, KODJABACHIAN L, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119(4):837-851.
[6] HINGORANI P, ZHANG W, GORLICK R, et al. Inhibition of Src Phosphorylation Alters Metastatic Potential of Osteosarcoma In vitro but not In vivo. Clin Cancer Res. 2009;15(10):3416-3422.
[7] CAI Y, CAI T, CHEN Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem. 2014;115(4):625-631.
[8] GUO M, CAI C, ZHAO G, et al. Hypoxia promotes migration and induces CXCR4 expression via HIF-1α activation in human osteosarcoma. PLoS ONE. 2014;9:e90518.
[9] SAVAGE SA, MIRABELLO L, WANG Z, et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet. 2013;45:799-803.
[10] DUNN SL, SOUL J, ANAND S, et al. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthritis Cartilage. 2016;24(8):1431-1440.
[11] WANG J, SONG Y. Single cell sequencing: a distinct new field. Clin Transl Med. 2017;6(1):10.
[12] BJORKLUND AK, FORKEL M, PICELLI S, et al. The heterogeneity of human CD127(+ ) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17(4):451-460.
[13] MEREDITH M, ZEMMOUR D, MATHIS D, et al. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16(9):942-949.
[14] POULIN JF, GAERTNER Z, MORENO-RAMOS OA, et al. Classification of midbrain dopamine neurons using single-cell gene expression profiling approaches. Trends Neurosci. 2020;43(3):155-169.
[15] SWANSON JB, DE MICHELI AJ, DISSER NP, et al. A single-cell transcriptional atlas identifies extensive heterogeneity in the cellular composition of tendons. BioRxiv. 2019. doi:10.1101/801266.
[16] RUBENSTEIN AB,SMITH GR,RAUE U,et al. Single-cell transcriptional profiles in human skeletal muscle.Sci Rep. 2020;10(1):229.
[17] JI Q, ZHENG Y, ZHANG G, et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann Rheum Dis. 2019;78(1):100-110.
[18] TANG F, BARBACIORU C, WANG Y, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature methods. 2009;6(5):377-382.
[19] SHAPIRO E, BIEZUNER T, LINNARSSON S, et al. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14(9):618-630.
[20] ZHANG XN, LI TQ, LIU F, et al. Comparative Analysis of Droplet-Based Ultra-High-Throughput Single-Cell RNA-Seq Systems. Molecular Cell. 2019;73(1):130-142.e5.
[21] MACOSKO EZ, BASU A, SATIJA R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202-1214.
[22] WANG Y, NAVIN NE. Advances and applications of single-cell sequencing technologies. Molecular Cell. 2015;58(4):598-609.
[23] OZSOLAK F, PLATT AR, JONES DR, et al. Direct RNA sequencing. Nature. 2009;461(7265):814-818.
[24] RODRIGUE S, MALMSTROM RR, BERLIN AM, et al. Whole genome amplification and de novo assembly of single bacterial cells. PloS One. 2009;4(9):e6864.
[25] ZONG CH, LU SJ, CHAPMAN AR, et al. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338(6114):1622-1626.
[26] ISCOVE NN, BARBARA M, GU M, et al. Representation is faithfully preserved in global cDNA amplified exponentially from sub-picogram quantities of mRNA. Nat Biotechnol. 2002;20(9):940-943.
[27] PARK J, SHRESTHA R, QIU C, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease.Science. 2018;360(6390):758-763.
[28] WU H, HUMPHREYS BD. The promise of single-cell RNA sequencing for kidney disease investigation. Kidney Int. 2017;92(6):1334-1342.
[29] CHAN CKF, SEO EY, CHEN JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285-298.
[30] MIZUHASHI K, ONO W, MATSUSHITA Y, et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018; 563(7730):254-258.
[31] CHAN CKF, GULATI GS, SINHA R, et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43-56.e21.
[32] MIZUHASHI K, NAGATA M, MATSUSHITA Y, et al. Growth plate borderline chondrocytes behave as transient mesenchymal precursor cells. J Bone Miner Res. 2019;34(8):1387-1392.
[33] TAKAHASHI A, NAGATA M, GUPTA A, et al. Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc Natl Acad Sci U S A. 2019;116(2):575-580.
[34] DEBNATH S, YALLOWITZ AR, MCCORMICK J, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133-139.
[35] ZHOU B, YUE R, MURPHY MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154-168.
[36] SACCHETTI B, FUNARI A, MICHIENZI S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324-336.
[37] BI W, DENG JM, ZHANG Z, et al. Sox9 is required for cartilage formation.Nature genetics. 1999;22:85-89.
[38] YAMASHIRO T, WANG XP, LI Z, et al. Possible roles of Runx1 and Sox9 in incipient intramembranous ossification. J Bone Miner Res. 2004; 19(10):1671-1617.
[39] ONO N, ONO W, NAGASAWA T, et al. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nature Cell Biology. 2014;16(12):1157-1167.
[40] AKIYAMA H, KIM JE, NAKASHIMA K, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102(41):14665-14670.
[41] KELLY NH, HUYNH NPT, GUILAK F. Single cell RNA-sequencing reveals cellular heterogeneity and trajectories of lineage specification during murine embryonic limb development. Matrix Biol. 2020;89:1-10.
[42] DECKER RS, KOYAMA E, PACIFICI M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5-10.
[43] LEE CH, LEE FY, TARAFDER S, et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 2015;125(7): 2690-2701.
[44] PRYCE BA, BRENT AE, MURCHISON ND, et al. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236(6):1677-1182.
[45] HUANG AH, LU HH, SCHWEITZER R. Molecular regulation of tendon cell fate during development. J Orthop Res. 2015;33(6):800-812.
[46] DISSER NP, GHAHRAMANI GC, SWANSON J, et al. Widespread diversity in the transcriptomes of functionally divergent limb tendons. J Physiol. 2020;598(8):1537-1550.
[47] HARVEY T, FLAMENCO S, FAN CM. A Tppp3Pdgfra tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis.Nat Cell Biol. 2019;21(12): 1490-1503.
[48] LESCROART F, WANG XN, LIN XH, et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science. 2018;359:1177-1181.
[49] HAREL I, NATHAN E, TIROSH-FINKEL L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16(6):822-832.
[50] CHAN S, SHI XZ, TOYAMA A, et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587-601.
[51] PENALOZA JS, PAPPAS MP, HAGEN HR, et al. Single-cell RNA-seq analysis of Mesp1-induced skeletal myogenic development. Biochem Biophys Res Commun. 2019;520(2):284-290.
[52] BARRUET E, GARCIA SM, STRIEDINGER K, et al. Functionally heterogeneous human satellite cells identified by single cell RNA sequencing. Elife. 2020;9:e51576.
[53] DE MICHELI AJ, SPECTOR JA, ELEMENTO O, et al. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skeletal Muscle. 2020;10:19.
[54] KANN AP, KRAUSS RS. Multiplexed RNAscope and immunofluorescence on whole-mount skeletal myofibers and their associated stem cells.Development. 2019;146(20):dev179259.
[55] GNOCCHI VF, WHITE RB, ONO Y, et al. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One. 2009;4(4):e5205.
[56] EINHORNA T, GERSTENFELD LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54.
[57] SALAZAR VS, GAMER LW, ROSEN V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12(4):203-221.
[58] MCBRIDE SH, MCKENZIE JA, BEDRICK BS, et al. Long bone structure and strength depend on BMP2 from osteoblasts and osteocytes, but not vascular endothelial cells. PLoS One. 2014;9(5):e96862.
[59] BOLANDER J, CHAI YC, GERIS L, et al. Early BMP, Wnt and Ca(2+)/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials. 2016;86: 106-118.
[60] BOLANDER J, JI W, GERIS L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur Cell Mater. 2016;31:11-25.
[61] BOLANDER J, HERPELINCK T, CHAKLADER M, et al. Single-cell characterization and metabolic profiling of in vitro cultured human skeletal progenitors with enhanced in vivo bone forming capacity.Stem Cells Transl Med. 2020;9(3):389-402.
[62] BOLANDER J, JI W, LEIJTEN J, et al. Healing of a Large Long-Bone Defect through Serum-Free In Vitro Priming of Human Periosteum-Derived Cells. Stem Cell Reports. 2017;8:758-772.
[63] PREIN C, WARMBOLD N, FARKAS Z, et al. Structural and mechanical properties of the proliferative zone of the developing murine growth plate cartilage assessed by atomic force microscopy. Matrix Biol. 2016;50:1-15.
[64] JIANG Y, TUANR S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206-212.
[65] BUCKLEY CD, BARONE F, NAYAR S, et al. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715-745.
[66] CROFT AP, CAMPOS J, JANSEN K, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246-251.
[67] STEPHENSON W, DONLIN LT, BUTLER A, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun. 2018;9(1):791.
[68] CAI SZ, MING BX, YE C, et al. Similar Transition Processes in Synovial Fibroblasts from Rheumatoid Arthritis and Osteoarthritis A Single-Cell Study. J Immunol Res. 2019;2019:4080735.
[69] MIZOGUCHI F, SLOWIKOWSKI K, KEVIN W, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. 2018;9(1):789.
[70] WADE SM, CANAVAN M, MCGARRY T, et al. Association of synovial tissue polyfunctional T-cells with DAPSA in psoriatic arthritis. Ann Rheum Dis. 2019;78(3):350-354.
[71] BERENBAUM F, MENG QJ. The brain-joint axis in osteoarthritis: nerves, circadian clocks and beyond. Nat Rev Rheumatol. 2016;12(9):508-516.
[72] SHAH KM,STERN MM,STERN AR,et al.Osteocyte isolation and culture methods. Bonekey Reports. 2016;5:838.
[73] TANAKA K-I, XUE Y, NGUYEN‐YAMAMOTO L, et al. FAM210A is a noveldeterminant of bone and muscle structure and strength.Proc Natl Acad Sci U S A. 2018;115(16):E3759-E3768.
[74] ADAM M, POTTER AS, POTTER SS. Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development. 2017;144(19):3625-3632.
[75] AYTURK U. RNA-seq in Skeletal Biology. Curr Osteoporos Rep. 2019; 17(4):178-185.
|