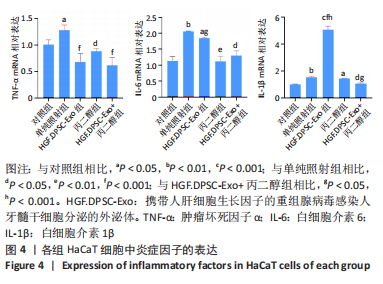
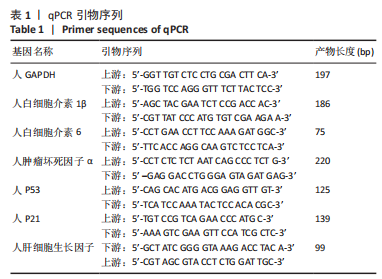
[1] ROSENTHAL A, ISRAILEVICH R, MOY R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81(2):558-567.
[2] BRAND RM, EPPERLY MW, STOTTLEMYER JM, et al. A Topical Mitochondria-Targeted Redox-Cycling Nitroxide Mitigates Oxidative Stress-Induced Skin Damage. J Invest Dermatol. 2017;137(3):576-586.
[3] NYSTEDT KE, HILL JE, MITCHELL AM, et al. The standardization of radiation skin care in British Columbia: a collaborative approach. Oncol Nurs Forum. 2005;32(6):1199-1205.
[4] HYMES SR, STROM EA, FIFE C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28-46.
[5] ZASADZIŃSKI K, SPAŁEK MJ, RUTKOWSKI P. Modern Dressings in Prevention and Therapy of Acute and Chronic Radiation Dermatitis-A Literature Review. Pharmaceutics. 2022;14(6):1204.
[6] YAO C, ZHOU Y, WANG H, et al. Adipose-derived stem cells alleviate radiation-induced dermatitis by suppressing apoptosis and downregulating cathepsin F expression. Stem Cell Res Ther. 2021;12(1):447.
[7] YI L, TIAN M, PIAO C, et al. The protective effects of 1,2-propanediol against radiation-induced hematopoietic injury in mice. Biomed Pharmacother. 2019;114: 108806.
[8] CRENSHAW MD, TEFFT ME, BUEHLER SS, et al. Determination of nicotine, glycerol, propylene glycol and water in electronic cigarette fluids using quantitative 1 H NMR. Magn Reson Chem. 2016;54(11):901-904.
[9] CARRER V, ALONSO C, PONT M, et al. Effect of propylene glycol on the skin penetration of drugs. Arch Dermatol Res. 2020;312(5):337-352.
[10] WANG PW, CHENG YC, HUNG YC, et al. Red Raspberry Extract Protects the Skin against UVB-Induced Damage with Antioxidative and Anti-inflammatory Properties. Oxid Med Cell Longev. 2019;2019:9529676.
[11] WU P, ZHANG B, SHI H, et al. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291-301.
[12] RAJENDRAN P, RENGARAJAN T, THANGAVEL J, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057-1069.
[13] SANADA F, TANIYAMA Y, AZUMA J, et al. Therapeutic Angiogenesis by Gene Therapy for Critical Limb Ischemia: Choice of Biological Agent. Immunol Endocr Metab Agents Med Chem. 2014;14(1):32-39.
[14] MOON SH, LEE CM, PARK SH, et al. Effects of hepatocyte growth factor gene-transfected mesenchymal stem cells on dimethylnitrosamine-induced liver fibrosis in rats. Growth Factors. 2019;37(3-4):105-119.
[15] PARK BW, JUNG SH, DAS S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020;6(13):eaay6994.
[16] WEN Q, ZHANG S, DU X, et al. The Multiplicity of Infection-Dependent Effects of Recombinant Adenovirus Carrying HGF Gene on the Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Int J Mol Sci. 2018;19(3):734.
[17] CHEN J, CHOPP M. Exosome Therapy for Stroke. Stroke. 2018;49(5):1083-1090.
[18] YANG X, REN H, GUO X, et al. Radiation-induced skin injury: pathogenesis, treatment, and management. Aging (Albany NY). 2020;12(22):23379-23393.
[19] NONAKA D, FUJIWARA R, HIRATA Y, et al. Metabolic engineering of 1,2-propanediol production from cellobiose using beta-glucosidase-expressing E. coli. Bioresour Technol. 2021;329:124858.
[20] MASSARSKY A, ABDEL A, GLAZER L, et al. Neurobehavioral effects of 1,2-propanediol in zebrafish (Danio rerio). Neurotoxicology. 2018;65:111-124.
[21] ZENG Z, LI S, BOEREN S, et al. Anaerobic Growth of Listeria monocytogenes on Rhamnose Is Stimulated by Vitamin B12 and Bacterial Microcompartment-Dependent 1,2-Propanediol Utilization. mSphere. 2021;6(4):e0043421.
[22] 中国人民解放军军事科学院军事医学研究院.丙二醇在制备用于预防肠型放射病及放射性肠炎的药物中的应用:CN201910230933.2[P]. 2019-05-28.
[23] SHAEFFER J, SCHELLENBERG KA. Polyethylene glycol as a protector against head and neck irradiation. Int J Radiat Oncol Biol Phys. 1984;10(12):2329-2333.
[24] MACHTAY M, SCHERPEREEL A, SANTIAGO J, et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81(2): 196-205.
[25] LIU D, KONG F, YUAN Y, et al. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int J Radiat Oncol Biol Phys. 2018;101(4):945-956.
[26] SUN J, ZHANG Y, SONG X, et al. The Healing Effects of Conditioned Medium Derived from Mesenchymal Stem Cells on Radiation-Induced Skin Wounds in Rats. Cell Transplant. 2019;28(1):105-115.
[27] WEN S, DOONER M, CHENG Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30(11):2221-2231.
[28] PIRYANI SO, JIAO Y, KAM AYF, et al. Endothelial Cell-Derived Extracellular Vesicles Mitigate Radiation-Induced Hematopoietic Injury. Int J Radiat Oncol Biol Phys. 2019;104(2):291-301.
[29] LIN Z, WU Y, XU Y, et al. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21(1):179.
[30] ZHAO H, LI Z, WANG Y, et al. Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front Cell Dev Biol. 2023;11:1029671.
[31] HU P, YANG Q, WANG Q, et al. Mesenchymal stromal cells-exosomes: a promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma. 2019;7:38.
[32] LU K, LI HY, YANG K, et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):108.
[33] LEI X, HE N, ZHU L, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid Redox Signal. 2021;35(11):849-862.
[34] CZYZ M. HGF/c-MET Signaling in Melanocytes and Melanoma. Int J Mol Sci. 2018; 19(12):3844.
[35] ZHAO Y, YE W, WANG YD, et al. HGF/c-Met: A Key Promoter in Liver Regeneration. Front Pharmacol. 2022;13:808855.
[36] NAKAMURA T, MIZUNO S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(6):588-610.
[37] CHEN W, WANG S, XIANG H, et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stem cells ameliorate acute lung injury partly mediated by hepatocyte growth factor. Int J Biochem Cell Biol. 2019;112:114-122.
[38] YARNOLD J, BROTONS MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149-161.
[39] DONG X, KONG F, LIU C, et al. Pulp stem cells with hepatocyte growth factor overexpression exhibit dual effects in rheumatoid arthritis. Stem Cell Res Ther. 2020;11(1):229.
[40] MENG HF, JIN J, WANG H, et al. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J Cell Mol Med. 2022;26(18):4745-4755.
[41] WANG H, SUN RT, LI Y, et al. HGF Gene Modification in Mesenchymal Stem Cells Reduces Radiation-Induced Intestinal Injury by Modulating Immunity. PLoS One. 2015;10(5):e0124420.
[42] GENG P, ZHANG Y, ZHANG H, et al. HGF-Modified Dental Pulp Stem Cells Mitigate the Inflammatory and Fibrotic Responses in Paraquat-Induced Acute Respiratory Distress Syndrome. Stem Cells Int. 2021;2021:6662831.
[43] USUNIER B, BROSSARD C, L’HOMME B, et al. HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis. Int J Mol Sci. 2021;22(4):1790.
[44] CUI HS, JOO SY, CHO YS, et al. Effect of Combining Low Temperature Plasma, Negative Pressure Wound Therapy, and Bone Marrow Mesenchymal Stem Cells on an Acute Skin Wound Healing Mouse Model. Int J Mol Sci. 2020;21(10):3675.
[45] JIAO Y, CAO F, LIU H. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022;123(5):376-386.
[46] PISTRITTO G, TRISCIUOGLIO D, CECI C, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8(4):603-619.
[47] LUO J, ZHANG M, HUANG H, et al. Matrilin-2 regulates proliferation, apoptosis and cell cycle during radiation-induced injury in HPAEpiC cell. Biochem Biophys Res Commun. 2017;485(3):577-583.
[48] SU X, UPADHYAY A, TRAN SD, et al. Cell-Free Therapies: The Use of Cell Extracts to Mitigate Irradiation-Injured Salivary Glands. Biology (Basel). 2023;12(2):305.
[49] CHEN H, CUI Z, LU W, et al. Association between serum manganese levels and diabetes in Chinese adults with hypertension. J Clin Hypertens (Greenwich). 2022; 24(7):918-927.
[50] ZHANG Y, DENG H, YANG Z, et al. Treatment of Radiation Bone Injury with Transplanted hUCB-MSCs via Wnt/β-Catenin. Stem Cells Int. 2021;2021:5660927.
|