中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (11): 1677-1682.doi: 10.3969/j.issn.2095-4344.2583
• 组织构建实验造模 experimental modeling in tissue construction • 上一篇 下一篇
富血小板血浆联合髓芯减压调控激素性股骨头坏死模型兔氧化应激反应
王 哲,李 炎,娄理想,陆 川,丁启龙,顾锡龙,李泽清
- 青海大学附属医院创伤骨科,青海省西宁市 810000
Platelet-rich plasma combined with core decompression regulates oxidative stress in a rabbit model of steroid-induced femoral head necrosis
Wang Zhe, Li Yan, Lou Lixiang, Lu Chuan, Ding Qilong, Gu Xilong, Li Zeqing
- Department of Traumatic Orthopedics, Affiliated Hospital of Qinghai University, Xining 810000, Qinghai Province, China
摘要:

文题释义:
激素性股骨头缺血性坏死:股骨头坏死又称股骨头无菌性坏死,或股骨头缺血性坏死,是由于多种原因导致 的股骨头局部血运不良,从而引起骨细胞进一步缺血、坏死、骨小梁断裂、股骨头塌陷的一种病变,激素性 股骨头坏死就是因为长时间使用激素而引起的一种股骨头坏死。
氧化应激损伤:氧化应激指机体在遭受各种内源性或外源性刺激时,体内高活性分子如活性氧自由基产生过多,氧化程度超出氧化物的清除,氧化系统和抗氧化系统失衡,从而导致组织损伤。正常情况下机体内活性氧自由基的产生和清除处于动态平衡,有害刺激可以打破这种平衡,机体会形成氧化应激状态。
背景:氧化应激在股骨头坏死损伤中发挥重要作用,富血小板血浆富含生长因子,可以加快骨折愈合,配合髓芯减压术能促进非创伤性股骨头坏死的恢复。
目的:探讨富血小板血浆联合髓芯减压术能否通过Keap1/Nrf2/HO-1信号通路抑制兔激素性股骨头坏死模型氧化应激反应。
方法:将40只实验新西兰兔随机分为正常组、模型组、对照组和富血小板血浆组,每组10只。除正常组外其他3组兔在无菌环境下建立激素性股骨头坏死模型,术后4周向富血小板血浆组动物股骨头内髓芯减压后注射植入0.4 mL 3%的富血小板血浆,对照组兔只进行髓芯减压术治疗,对照组与模型组兔正常饲养。14周后苏木精-伊红染色观察各组兔股骨头骨髓腔内病理学变化和骨陷窝空缺率,检测各组兔血清中总抗氧化能力、超氧化物歧化酶、谷胱甘肽过氧化物酶、还原型谷胱甘肽及丙二醛等氧化应激指标活性,TUNEL检测股骨头组织内骨细胞凋亡情况,免疫荧光检测股骨头组织内Keap1、Nrf2分布,Western Blot检测股骨头组织内Keap1、Nrf2、HO-1蛋白表达。实验方案经青海大学附属医院动物实验伦理委员会批准(批准号为qhdx-201908374)。
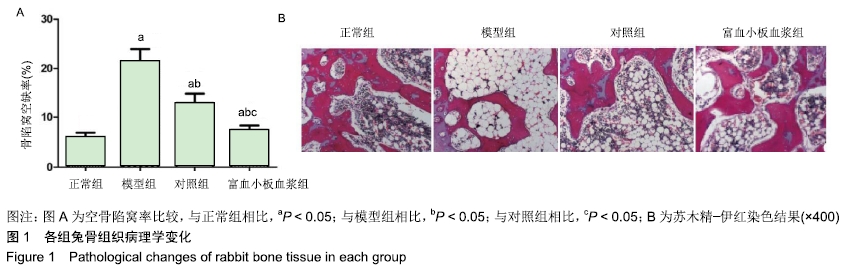
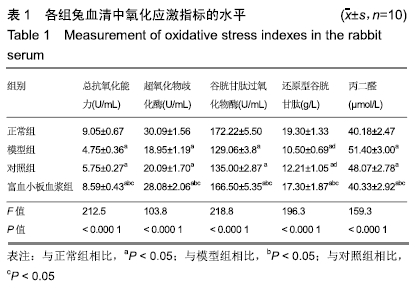
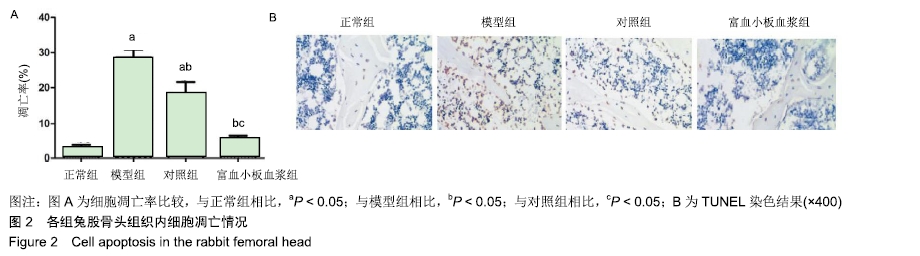
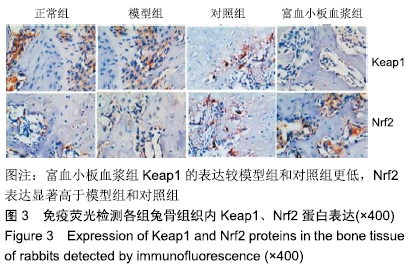
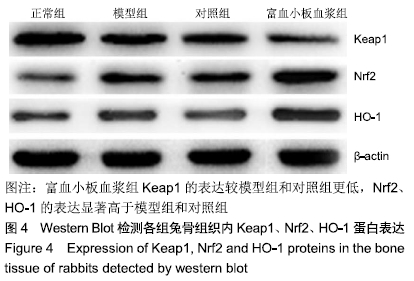
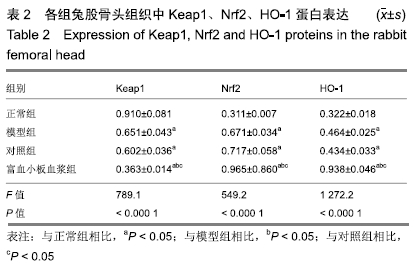
结果与结论:①与正常组相比,模型组骨组织内骨小梁变细,结构紊乱;对照组较模型组有所改善,骨小梁结构得到恢复,空骨陷窝减少(P < 0.05),富血小板血浆组经富血小板血浆联合髓芯减压治疗较对照组得到进一步改善,骨小梁结构更加完善,空骨陷窝进一步减少(P < 0.05),与正常组无显著差异(P > 0.05);②模型组血清中总抗氧化能力、超氧化物歧化酶、谷胱甘肽过氧化物酶和还原型谷胱甘肽的含量均显著低于正常组(P < 0.05),而丙二醛浓度显著高于正常组(P < 0.05);对照组以上指标稍有改善,但与模型组比较差异不显著(P > 0.05);富血小板血浆以上氧化应激指标较模型组和对照组明显改善(P < 0.05);③模型组股骨头组织内Keap1蛋白表达显著低于正常组(P < 0.05),Nrf2、HO-1蛋白表达显著高于正常组(P < 0.05);富血小板血浆组股骨头组织内Keap1的表达较模型组和对照组低(P < 0.05),Nrf2、HO-1的表达显著高于模型组和对照组(P < 0.05);④结果提示,富血小板血浆能有效抑制兔激素性股骨头坏死过程中氧化应激反应,该作用可能是通过激活Keap1/Nrf2/HO-1信号通路活性而发生的。
ORCID: 0000-0002-8371-2665(王哲)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: