中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (15): 2437-2444.doi: 10.12307/2024.268
• 生物材料综述 biomaterial review • 上一篇 下一篇
基于支架和无支架策略在生长板软骨再生治疗中的应用
郭若宜,庄汉杰,陈修宁,贲雨龙,范民杰,王亦维,郑朋飞
- 南京医科大学附属儿童医院骨科,江苏省南京市 210000
-
收稿日期:2023-03-13接受日期:2023-04-28出版日期:2024-05-28发布日期:2023-09-23 -
通讯作者:郑朋飞,副教授,副主任医师,南京医科大学附属儿童医院骨科,江苏省南京市 210000 -
作者简介:郭若宜,男,1998年生,福建省厦门市人,汉族,主要从事生长板损伤后软骨再生的治疗研究。 -
基金资助:江苏省重点研发计划项目(BE2019608),项目负责人:郑朋飞;中国博士后研究基金(2022M721685),项目负责人:郑朋飞;江苏省卫生健康委医学科研项目(2020158),项目负责人:郑朋飞;南京市科技发展计划医疗卫生国际联合研发项目(2020),项目负责人:郑朋飞;转化医学国家重大科技基础设施(上海)开放课题项目(TMSK-2021-304),项目负责人:郑朋飞;南京市医学科技发展重点项目(ZKX18041),项目负责人:郑朋飞
Application of scaffold-based and scaffold-free strategy for treatment of growth plate cartilage regeneration
Guo Ruoyi, Zhuang Hanjie, Chen Xiuning, Ben Yulong, Fan Minjie, Wang Yiwei, Zheng Pengfei
- Department of Orthopedics, Children’s Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China
-
Received:2023-03-13Accepted:2023-04-28Online:2024-05-28Published:2023-09-23 -
Contact:Zheng Pengfei, Associate professor, Associate chief physician, Department of Orthopedics, Children’s Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China -
About author:Guo Ruoyi, Department of Orthopedics, Children’s Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China -
Supported by:Jiangsu Provincial Key Research and Development Program, No. BE2019608 (to ZPF); Postdoctoral Research Foundation of China, No. 2022M721685 (to ZPF); Jiangsu Health Commission Medical Research Program, No. 2020158 (to ZPF); Nanjing Science and Technology Development Plan Medical and Health International Joint Research and Development Project, No. 2020 (to ZPF); National Facility for Translational Medicine (Shanghai) Open Program, No. TMSK-2021-304 (to ZPF); Key Program of Nanjing Medical Science and Technology Development, No. ZKX18041 (to ZPF)
摘要:
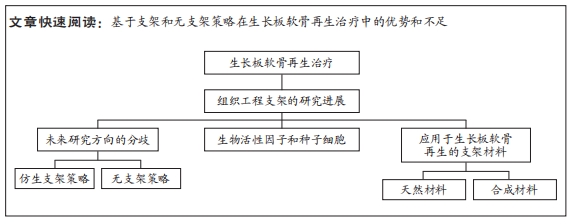
文题释义:
生长板:又称骺板,是儿童未成熟长骨末端控制骨骼纵向生长的软骨区域,同时也是儿童骨骼中最脆弱的区域。由于生长板软骨再生能力差以及未成熟骨骼具有动态特性,一旦发生损伤,生长板损伤后缺损部位易发生骨组织修复,进而形成“骨桥”,并导致生长阻滞、成角或旋转畸形,造成严重远期并发症。三维支架:通常用于组织工程领域的药物输送、细胞移植以及细胞行为和材料研究。目前组织工程学的三维支架通常是采用具有生物相容性和可生物降解的材料,目的是构建一个合适的微环境,为最佳的细胞生长和功能提供相应的支持。
背景:组织工程是一种理想的生长板再生治疗方式,然而目前大多数的再生组织工程研究都是建立在传统支架策略的基础之上,随着传统支架的局限性逐渐显露,研究的方向也逐渐多样化。
目的:总结基于支架和无支架策略在生长板软骨再生治疗中的应用以及各自的优势和不足。方法:检索PubMed、Wiley、Elsevier数据库收录的相关文献,英文检索词为“growth plate injury,regeneration,tissue engineering,scaffold,scaffold-free,biomimetic,cartilage”。检索时限为1990-2023年,最终纳入104篇文献进行综述。
结果与结论:仿生支架策略是通过模拟生长板独特的组织结构最大程度上还原每个区域的细胞组成、生物信号和独特力学性能,进而构建能促进组织再生的仿生微环境,因此,仿生支架的设计是尽可能地从成分、结构和力学性能上模拟原生生长板,虽然取得一定的成效,但仍存在再生效果不稳定的问题。无支架策略认为支架的局限性会对再生治疗产生不利影响,因此,无支架构建物的设计是尽可能地依赖于细胞自身产生和维持细胞外基质的能力,不干扰细胞-细胞间的信号,不引入外源性物质,但存在稳定性较差、机械强度低,操作难度较大等问题。仿生策略和无支架策略的出发点不同且均存在各自的优缺点,但它们对于生长板软骨再生均能产生积极的作用。因此,后续的研究不论是采取仿生策略或无支架策略,都将聚焦于对现有技术的不断优化,以期实现有效的生长板软骨再生治疗。
https://orcid.org/0009-0008-3149-9605(郭若宜)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
郭若宜, 庄汉杰, 陈修宁, 贲雨龙, 范民杰, 王亦维, 郑朋飞. 基于支架和无支架策略在生长板软骨再生治疗中的应用[J]. 中国组织工程研究, 2024, 28(15): 2437-2444.
Guo Ruoyi, Zhuang Hanjie, Chen Xiuning, Ben Yulong, Fan Minjie, Wang Yiwei, Zheng Pengfei. Application of scaffold-based and scaffold-free strategy for treatment of growth plate cartilage regeneration[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2437-2444.
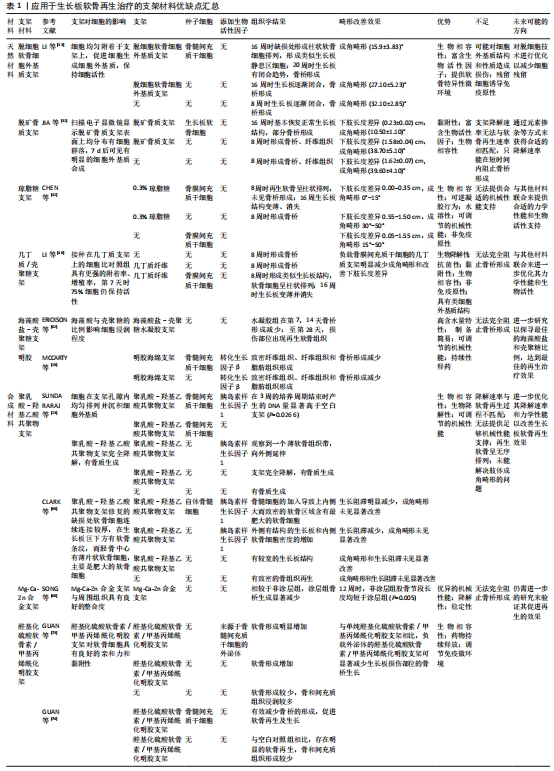
为了改善生长板修复的微环境,许多研究利用生物活性因子如胰岛素样生长因子1、成纤维细胞生长因子2、转化生长因子β等来促进细胞增殖和成软骨分化。胰岛素样生长因子1是一种被广泛研究的合成代谢生长因子,在软骨稳态和修复中发挥至关重要[22]。SUNDARARAJ等[23]使用无细胞聚乳酸-羟基乙酸支架局部递送胰岛素样生长因子1来治疗兔胫骨近端的生长板损伤,空白对照组和单独支架组中仅观察到骨桥形成,而负载胰岛素样生长因子1的支架植入后可以观察到明显的软骨再生,表明胰岛素样生长因子1在促成软骨和防止骨桥形成中发挥重要作用。成纤维细胞生长因子家族对多种细胞类型的分化、增殖、迁移和生长十分重要,是软骨再生活动中的重要调节因素[24]。在生长板损伤后的成纤维期,成纤维细胞生长因子2可以刺激骨祖细胞和间充质干细胞的增殖、迁移以及抑制软骨细胞肥大[25]。COLEMAN等[26]的研究发现,与转化生长因子β单独处理相比,成纤维细胞生长因子2和转化生长因子β共同处理会使骨髓间充质干细胞产生更多糖胺聚糖,这提示生物活性因子的联合应用能更好地改善再生微环境并提升治疗效果。转化生长因子β是一类结构保守、功能多样的生物活性因子,在间充质干细胞向软骨分化的过程中发挥重要作用[27]。MCCARTY等[28]将含有自体骨髓间充质干细胞和转化生长因子β的明胶海绵支架植入绵羊胫骨近端生长板缺损,结果表明相较于对照组,含有转化生长因子β的支架能明显抑制生长板损伤部位的骨桥形成。此外,基质细胞衍生因子1和转化生长因子β3与生长板组织再生密切相关,基质细胞衍生因子1能促进间充质干细胞向缺陷部位迁移[29],而转化生长因子β3作为软骨形成因子能通过Smad信号通路增强Sox9基因的转录活性,诱导间充质干细胞向成软骨分化[30],因此,两者可以在生长板损伤治疗中协同发挥修复作用。综上,生物活性因子和种子细胞是组织工程中的核心要素,在组织工程策略治疗生长板损伤中发挥着重要作用。
2.2 支架材料在生长板软骨再生治疗中的应用 组织工程支架在提供力学支持的同时也会改变损伤部位的微环境,进而影响生长板软骨的再生效果,因此随着组织工程研究的进展,支架材料本身的特性也越发受到重视,如理化性能和生物学性能等。目前的支架材料依据其来源可以分为天然材料和合成材料。
2.2.1 天然材料
细胞外基质支架:细胞外基质是自然界理想的生物支架材料,具有良好的生物相容性、生物活性和生物安全性[31],因此,基于细胞外基质的生物支架适合用于组织的再生修复治疗。脱细胞软骨细胞外基质作为细胞外基质衍生物之一具有良好的生物相容性,并能够提供软骨再生的特异性微环境,是理想的软骨再生支架材料之一[32]。LI等[33]设计了一种具有优异生物力学性能的定向脱细胞细胞外基质支架,用于生长板缺损部位的软骨再生治疗显示了良好的生长板损伤修复潜力。然而,脱细胞细胞外基质支架制备过程中存在对细胞外基质原有结构和性质造成损害的可能性,以及制备过程中细胞残留成分可能会诱导免疫反应[34],因此未来还需要进一步的研究并进行技术上的优化,以减少脱细胞过程中细胞残留所导致的不良反应。此外,脱矿骨基质支架由含有多种生物活性因子的胶原组成,而脱矿骨基质的制备方法是由URIST发明[35]。JIN等[36]将兔生长板软骨细胞植入脱矿骨基质支架,软骨细胞可以黏附在脱矿骨基质支架表面并继续进行增殖,表明支架具有良好的黏附性和生物相容性;支架的多孔结构允许细胞因子和营养物质等通过孔隙提供给支架内的软骨细胞,但脱矿骨基质支架只能在短时间内阻止骨桥的形成,之后由于支架被吸收后骨和纤维组织长入缺损处,出现严重的成角畸形。脱矿骨基质可能需要通过掺杂元素或其他途径来改变其降解速率,使其能在生长板软骨细胞再生过程中提供合适的再生微环境,防止支架过早降解而影响再生效果。细胞外基质及其衍生物展现出促进生长板软骨再生的潜力,但理想组织工程支架的力学性能应与目标再生组织的力学性能相匹配,而单纯的细胞外基质本身机械性能不足,易造成塌陷,导致修复结果不理想[37]。因此,后续研究需要继续探寻合适的细胞外基质来源并优化支架的力学性能。
明胶支架:明胶是胶原部分水解后的产物,是一种常见的高分子材料,具有低免疫原性及良好的生物相容性和生物降解性。通过改性或与其他生物材料的结合,明胶能用于组织工程领域中药物输送的载体[38]。明胶不仅能促进成骨细胞的黏附和增殖,还能增加细胞外基质的分泌[39]。MCCARTY等[28]将自体骨髓间充质干细和转化生长因子β加入明胶海绵支架中,能有效抑制骨桥的形成。然而,明胶的机械强度相对较低,需要结合其他生物材料或进行改性以增强其理化性能,或是赋予其新的生物学作用,以便更好地应用于生长板损后的再生修复治疗中[40]。
琼脂糖支架:琼脂糖是一种天然多糖聚合物,具有良好的生物相容性、水溶性、非免疫原性及可调节的机械性能等特点,已被广泛应用于组织工程和再生医学的应用中[41]。CHEN等[42]将负载间充质干细胞的琼脂糖支架植入生长板损伤部位,结果显示能部分缓解兔胫骨的生长阻滞,但长期仍存在成角畸形和长度差异的问题,这或许是琼脂糖机械性能不足所造成的。BEEN等[43]采用蛋壳膜结合琼脂糖,其中蛋壳膜结构和细胞外基质相似,内含大量胶原蛋白,具有良好的生物相容性和生物降解性,但由于单纯蛋壳膜或单纯琼脂糖的机械性能不足,实验中通过将不溶性蛋壳膜中胶原蛋白的二硫键切断使其转化为水溶性,然后与琼脂糖结合制备蛋壳膜/琼脂糖复合支架;在体外实验中,复合支架促进软骨细胞增殖和细胞外基质的合成和分泌。因此,琼脂糖与其他材料联合来提供合适的机械性能和生物活性支持,是未来改善生长板软骨再生治疗效果的一种潜在方式。
几丁质/壳聚糖支架:几丁质是节肢动物和真菌外骨骼的主要成分,而壳聚糖是几丁质的去乙酰化形式[44]。几丁质/壳聚糖具有优异的生物降解性、抗菌性、黏附性、生物相容性及非免疫原性等特点,被广泛应用于组织的再生治疗[45]。LI等[46]将负载骨膜间充质干细胞的几丁质支架植入生长板缺损,有效地矫正下肢长度差异并减缓生长阻滞。骨膜间充质干细胞在几丁质支架中的存活率和增殖性更高,这可能与几丁质的组成成分在结构上类似于软骨细胞外基质的主要成分——糖胺聚糖有关。PLANKA等[47]设计了一种由Ⅰ型胶原纤维和壳聚糖组成的新型分层支架。该支架具有良好的机械性以及水动力播种的特性,能够对种子细胞起到良好的保护作用;同时,外围植入骨髓间充质干细胞和中心植入软骨细胞的分层技术,能有效预防骨桥形成和减少成角畸形;将支架植入后,结果显示生长板缺损处出现类似透明软骨的组织,呈现出生长板典型的柱状结构,这表明该支架具有促进生长板软骨再生的潜力。此外,ERICKSON等[48]制备的京尼平-壳聚糖微凝胶在体内生长板软骨缺损中也具有阻止早期骨桥形成的作用,表明京尼平-壳聚糖微凝胶在生长板软骨再生治疗中转化应用的潜力;然而,负载促软骨形成因子的支架组与空白支架组的生长板软骨再生效果之间无明显差异,因此后续可能需要进一步测试和改良优化支架的生物学性能。
海藻酸盐支架:海藻酸是一种高含水量、高孔隙结构、可调节黏度的生物相容性材料,具有易成凝胶的特性,其高孔隙结构有助于细胞的黏附、生长以及物质交换[49]。此外,海藻酸盐具有成本效益高、无细胞毒性、修饰简单、易于交联等特点[50]。ERICKSON等[48]用海藻酸盐水凝胶包裹生长板软骨细胞构建一种三维(3D)体外培养模型,在水凝胶中软骨细胞表现出高增殖能力,软骨基质沉积和低水平的软骨细胞肥大。先前的研究发现,甲状旁腺激素相关蛋白与印第安刺猬蛋白构成的信号通路调控生长板软骨细胞的增殖、分化与表型维持,在生长板再生过程中起着极为关键的作用[51-53],该模型能保留调节生长板软骨的甲状旁腺激素相关蛋白-印第安刺猬蛋白信号反馈环;其中,外源性甲状旁腺激素相关蛋白能刺激模型内细胞增殖并抑制细胞肥大,而印第安刺猬蛋白信号刺激软骨细胞肥大并形成生长板肥大区,因此,该培养模型还原生长板软骨的分层结构。未来可以进一步构造生长板软骨类似物,培养特定几何形状的软骨,以满足不同缺损的个性化需求[54]。此外,STURTIVANT等[55]使用抗冻蛋白修饰海藻酸盐的孔隙结构制备一种具有定向排列孔隙的支架,对细胞活力和功能有积极影响。
海藻酸盐-壳聚糖支架:海藻酸盐因其携带负电荷和具有较差的力学性能[56-57],使其应用范围受限。然而,带负电荷的海藻酸盐和带正电荷的壳聚糖可自然结合形成海藻酸盐-壳聚糖聚电解质复合物水凝胶,水凝胶的亲水特性能模拟软骨组织中存在的高含水量(高达80%)条件[58],而且可以通过调节影响微环境的参数来指导干细胞的命运,因而在组织工程中具有特别的意义[59]。STAGER等[60]发明了一种制备聚电解质复合物水凝胶的简易方法,将聚合物溶液在注射器之间来回强烈混合以产生海藻酸盐-壳聚糖聚电解质复合物水凝胶支架,具有力学性能可控和可持续性释药的优点,支架中低海藻酸分子质量可以导致人间充质干细胞的成骨分化潜力降低,而存储模量在1-10 kPa范围内的支架更有可能引导人间充质干细胞的成软骨分化,因此调控聚合物的分子质量和储存模量可以作为海藻酸盐-壳聚糖聚电解质复合物水凝胶控制干细胞分化的特异性信号线索。ERIKCSON等[61]发现海藻酸盐-壳聚糖聚电解质复合物水凝胶的释药速率与组分间的静电相互作用相关,海藻酸盐比例高则导致快速释药,而壳聚糖比例高则导致缓释药物,当负载抗血管生成药物的支架植入缺损后,快速释放模式能更好地靶向抑制骨桥形成。此外,ERIKCSON等[62]的另一项研究发现,通过优化海藻酸盐-壳聚糖聚电解质复合物水凝胶的降解速率可以影响干细胞命运,进而改善生长板软骨损伤后的再生效果,减少骨桥形成,因此可作为生长板损伤的治疗选择。未来仍需进一步研究不同海藻酸盐和壳聚糖比例对水凝胶释药速度、降解速率等的影响,并进一步探究其指导干细胞命运的机制,设计出最佳的组织工程支架。
2.2.2 合成材料
聚乳酸-羟基乙酸共聚物支架:聚乳酸-羟基乙酸共聚物具有优异的生物相容性、生物降解性以及可调节的机械性能[63],使用聚乳酸-羟基乙酸共聚物微球的药物递送装置已经获得FDA批准。SUNDARARAJ等[23]通过聚乳酸-羟基乙酸共聚物支架局部递送胰岛素样生长因子1来治疗生长板损伤,支架植入后的轻微溶胀有利于增强支架与周围组织之间整合,且均匀分布的孔隙结构则有助于间充质干细胞迁移进入支架内,结果显示支架能促进软骨再生,但再生软骨内软骨细胞呈无序排列的状态,这或许与支架未能提供足够的机械支撑及降解过快造成支撑时间较短有关。此外,聚乳酸-羟基乙酸共聚物支架可为间充质干细胞的增殖和成软骨分化创造必要的微环境[64]。CLARK等[65]将负载胰岛素样生长因子1的聚乳酸-羟基乙酸共聚物支架植入缺损部位,结果发现缺损部位的软骨细胞形成增加且对邻近生长板也起到保护作用,但肢体的成角畸形并未得到明显改善。综上,聚乳酸-羟基乙酸共聚物支架可以运用于生长板再生治疗,未来应考虑加强其机械性能以赋予支架更好的力学支撑作用。
Mg-Ca-Zn合金支架:镁基材料具有生物降解性和生物相容性,不易引起炎症反应,而且镁基材料的力学性能与皮质骨非常相似,故而导致的应力屏蔽反应比传统骨科填充材料小[66]。然而,镁基材料在快速降解过程中形成的氢气和水会导致植入物降解增快[67]。SONG等[68]通过表面改性的方法设计了一种含有等离子电解质氧化涂层的Mg-Ca-Zn合金,与未覆涂层的合金相比,涂层Mg-Ca-Zn合金的降解速度明显较慢且均匀、氢气生成减少、表面机械性能和稳定性增加,且降解时局部组织中Mg2+、Ca2+和Zn2+的浓度维持在正常范围,体内实验也表明植入物未引起明显的免疫反应,在整个过程中与周围组织结合较紧密,表现出优异的机械性能、降解能力和稳定性,最终,缺损处仅形成小骨桥且生长板的功能未受到植入物的干扰。因此,含有等离子电解质氧化涂层的Mg-Ca-Zn合金在生长板损伤的治疗中具有一定的发展潜力。然而,目前镁合金用于生长板再生的研究较少,更多研究聚焦于骨缺损的修复[69],因此,仍需进一步的研究来验证其促进生长板软骨再生的作用。
醛基化硫酸软骨素/甲基丙烯酰化明胶水凝胶支架:甲基丙烯酰化明胶水凝胶具有天然细胞外基质相似的特性,同时还具有优良的生物性能和可调节的物理性能[70]。硫酸软骨素是一种糖胺聚糖,主要存在于生物组织的细胞外基质中,表现出良好的生物相容性和诱导软骨形成的能力[71-72]。GUAN等[73]通过动态席夫碱键将醛基化硫酸软骨素与甲基丙烯酰化明胶相结合,形成具有优异生物相容性的类细胞外基质水凝胶,该水凝胶的双交联网络确保所负载外泌体的持续释放,调节损伤部位的免疫微环境、诱导抗炎因子的高表达、抑制损伤部位的骨桥形成,同时为骨髓间充质干细胞构建最佳的再生微环境,增强干细胞的成软骨分化,促进软骨再生。因此,该水凝胶可以实现负载药物或因子的持续释放,有利于生长板损伤后的软骨再生,但其临床转化的可行性还需进一步评估[74]。综上,目前大多数使用合成材料的研究都是在动物模型上进行[75],其安全性和临床转化应用能力有待进一步验证(表1)。
2.3 基于仿生理念的生长板软骨再生治疗策略 最近,仿生理念为软骨缺损提供了一种有前景的再生治疗策略:通过模拟关节软骨的分层结构最大程度上还原每个区域的细胞组成、生物信号及力学特性,进而构建组织再生的仿生微环境。WANG等[76]通过模仿成熟关节软骨的结构设计、化学线索和力学性能制备了一种双层仿生软骨支架,这种支架具有与天然软骨相似的组织结构和力学性能,可以引导骨髓间充质干细胞的形态、定向、增殖和分化,结果显示该仿生软骨支架可促进软骨组织再生。该实验通过设计一种与原生组织类似的结构来引导种子细胞植入后的行为,提供一种适合再生的微环境,这给予人们制备仿生支架用于生长板再生修复的灵感:通过模拟生长板的结构特征来尽可能构建适合组织再生的微环境,增强生长板损伤治疗效果,因此,理解生长板的特殊结构对于仿生结构的设计至关重要。
2.3.1 生长板的特性 先前提到生长板软骨细胞依据组织形态和功能分为3个区:静息区、增殖区和肥大区。生长板形态不均匀,不同区域具有其特有的机械性能[77],因此,在模拟原生生长板软骨组织结构时应充分考虑其机械性能的变化。先前研究表明与其他区域相比,生长板静息区具有较高的硬度和较低的渗透性,呈现出致密的透明软骨结构以及相对较少的静息软骨细胞;增殖区的硬度比静息区低约30%,而渗透率高约4倍,增殖区中的软骨细胞呈柱状排列,而增殖区与肥大区的力学性能并无特殊差异[78]。ECKSTEIN等[77]发现,生长板上肥大区到下肥大区之间的硬度改变可能为引导软骨细胞发生软骨内成骨提供了机械信号。生长板机械性能的空间差异在一定程度上是由细胞外基质组成不同所造成的,例如静息区因存在致密胶原结构,因而成为最坚硬的区域,因此生长板不同区带间存在一种梯度变化,这在设计仿生结构时需要格外注意。此外,生长板受复杂的局部力学因素和分子环境的影响,如动态压缩力已被证明能刺激生长板软骨生长,而剪切和静水压力更有可能推动软骨向成骨分化[79],这提示适当的机械刺激对于正常生长板的结构和功能具有重要作用。对静息区和增殖区给予机械负荷可诱导细胞纵向排列以维持生长板柱状结构的形成,并驱动纵向生长[80]。同时,生长板内机械调节信号(静水应力和最大拉伸应力)存在梯度变化,增殖区软骨细胞较大的机械调节信号能维持软骨状态,防止软骨细胞分化以及提供有利于细胞分裂的条件;机械调节信号沿软骨长轴向肥大区逐渐减小,并逐渐提供利于成骨分化的条件[81]。因此,生长板的独特结构以及机械性能对于正常生长功能的维持至关重要,而忽略了这些关键因素可能会造成再生修复的效果不理想。
2.3.2 仿生的技术支持 制备仿生支架除了要了解生长板的原生结构和功能外,还需要有相应技术的支持。生物3D打印技术能够确保细胞、材料、生物活性分子和机械结构精密结合起来,是再生医学中最有潜力的技术方法[82]。该技术能够在微尺度上精确控制支架微观结构和成分,并且可以在解剖学上密切模仿生物组织的几何结构而实现多层次组织结构的设计。与传统的组织工程支架制备方法相比,生物3D打印技术允许在3D空间中精确沉积生物材料和细胞,从而构建更加复杂和精确的空间关系[83],因此3D生物打印技术能够提供一种在体外模拟原生组织生态位的理想方法[84]。
适用于骨或软骨组织工程的常用3D打印技术包括3D绘图/直接墨水书写、立体平版印刷、选择性激光烧结、选择性激光熔融、熔融沉积建模、基于喷墨/挤压的生物打印以及立体光刻数字光处理技术[85-86]。此外,通过将3D三维打印、计算机辅助设计技术和影像学资料相结合来设计出具有个性化的支架结构也是一种有意义的方向[87]。然而,生长板损伤后的病理过程在时间和空间上都存在着动态变化,为了使得组织工程植入物对于动态变化过程中的各种刺激进行响应并高度适应需要将动态变化的理念引入3D打印技术之中,因而产生了将“时间”维度与3D打印相结合形成“4D打印”的新思路。4D打印技术可以在组织支架内实现随时间变化的物理变化或还原原生组织的动态生物行为[88],该技术允许打印材料在受到外部刺激(如温度、水、磁性和pH值)时改变其物理形式或功能。传统3D打印只考虑了打印物体的初始刚性和静态状态,而4D打印的支架具有在内部和外部刺激下自发地随时间变化的特性,从而更好地匹配生长板缺损内部复杂的生理环境,且其独特的响应性功能和形状转换能力可能有助于更准确地模拟原生组织的动态情况,从而实现真正意义上的仿生。然而,材料和组织之间确切的生物学相互作用尚未明确,技术支持也还处在初步阶段,但具有进一步发展的潜力和意义。
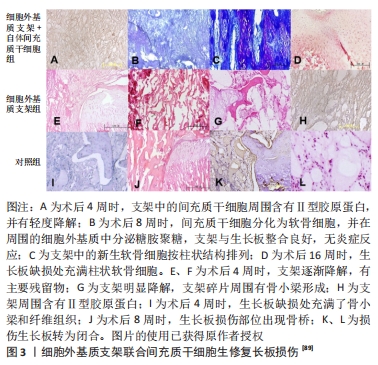
2.3.3 生长板仿生支架的应用 目前只有少数研究将仿生支架运用于生长板损伤的治疗。LI等[89]提出支架结构的设计应模仿生长板的天然软骨,即具有与其机械和生物功能相关的软骨细胞定向结构,因此,他们开发了一种能够模拟天然生长板柱状结构的细胞外基质衍生的脱细胞支架,支架呈现出一种横向平面的均匀孔径和纵向平面的仿生柱状结构,间充质干细胞在其中以一种类似于天然软骨细胞的柱状结构进行分布;支架中的定向微管结构能诱导间充质干细胞迁移到支架的核心,并且该结构可能可以增强支架的机械性能,以保护细胞在沉积足够的细胞外基质之前免受早期临界压缩的损害;同时,支架具有极好的生物相容性,其提取物对间充质干细胞无毒性;最终生长板损伤修复结果显示,该支架促进了新生软骨细胞的再生并阻止骨桥形成,从而减少了成角畸形和长度差异,虽然不能完全预防成角畸形和下肢短缩畸形,但细胞外基质衍生的定向生长板仿生支架展现出修复受损生长板的潜力(图3)。
YU等[85]设计了一种3D打印仿生复合材料,能满足生长板的机械需求,并包含能够支持软骨形成的生物活性生态位。该研究首先对兔生长板的力学性能进行表征,通过得到的模量数据来构建与原生组织相似性能的仿生支架。其中,仿生支架需要运用立体光刻数字光处理技术来构建,与其他3D打印技术相比,立体光刻数字光处理3D打印技术不需要悬垂特征的支撑材料且打印具有更高的分辨率,故而允许更复杂的设计。该研究运用立体光刻数字光处理 3D打印技术结合聚乙二醇、基树脂来创建具有垂直柱状的刚性3D打印结构,随后将该支架与软骨模拟水凝胶结合以形成仿生复合材料并植入损伤部位。在体外,这种仿生复合材料可以诱导间充质干细胞成软骨分化以及促进细胞外基质的生成;体内实验结果显示与对照组相比,植入后肢体生长能力得以部分恢复,然而,复合材料组仅在损伤处形成有限的软骨样组织。
总之,目前有少数用于生长板损伤修复的仿生支架,先前提到的两项研究都通过构建含有垂直柱状结构的支架来模仿生长板的柱状结构,结果表明仿生结构在促进生长板再生方面取得了初步成效,但也存在治疗效果不稳定性的问题。因此,可能需要进一步筛选支架的组分、优化支架的结构以及合理运用种子细胞和生物活性因子,以增强生长板软骨再生能力。此外,未来临床应用时应当考虑到更加复杂的问题,例如:生长板损伤后修复的过程中的炎症环境以及细菌感染、不同年龄阶段的生长板机械性能不同等。未来在设计仿生复合支架时可以结合计算机辅助技术等先进技术对生长板的机械性能和不同区域的组织结构进行表征,进而设计出一种具有分层结构的个性化仿生支架,充分模拟原生生长板,实现高效的生长板再生修复。
2.4 基于无支架理念的生长板软骨再生治疗策略 组织工程支架采用具有生物相容性、生物降解性和合适机械性能的天然或合成材料,同时结合先进的3D打印技术,旨在创造有效的细胞递送策略和提供有效的3D结构支持,已应用于生长板软骨再生治疗中,但植入支架的尺寸、孔隙率、形状及理化特性会对植入后的免疫反应和再生效果有所影响[62,69]。许多支架难以模拟原生组织条件,进而导致支架与原生组织的力学性能不匹配[90],因此,目前仍在积极开发合适的材料来克服支架存在的局限性。由于支架的缺陷会对组织再生治疗的效果产生不可忽视的影响,也有研究将思路转向无支架策略在组织再生治疗中的应用。与基于支架的再生治疗策略相比,无支架策略更依赖于细胞产生和维持自身细胞外基质的能力,不干扰细胞-细胞信号、细胞迁移或植入后的小分子扩散,目前被认为能最大程度还原体内的软骨环境[91-92]。
2.4.1 细胞片技术 无支架策略的代表——细胞片,具有细胞密度高和细胞分布均匀的特点,而且能有效地保存细胞-细胞以及细胞-细胞外基质间的相互作用[93]。沉积的细胞外基质具有自黏性,能有效促进植入细胞片与原生组织的结合[94];此外,细胞外基质富含生物活性因子和营养分子,同时可以保护植入细胞。通过结合温度响应性细胞培养技术,可实现模拟原生组织微结构的设计[93]。细胞片技术也能避免支架的限制和潜在问题[95-96]。DING等[97]对比软骨细胞片和负载软骨细胞的聚乙醇酸/聚乳酸支架植入体内后的稳定性,软骨细胞片在体内培养24周时表现出相对更稳定和成熟的软骨再生结构,而且无明显的炎症反应,而聚乙醇酸/聚乳酸支架因其降解不完全而导致植入处明显的炎症反应,进而阻碍软骨形成并导致细胞死亡,提示细胞片技术在生物相容性方面具有一定程度上的优势。值得一提的是,SATO等[98]将热响应培养皿制成的软骨细胞片与传统的骨髓刺激疗法结合并在8例患者身上进行了临床测试,结果证实了细胞片技术在临床测试中具有安全性和有效性。在生长板软骨再生领域,GüLTEK?N等[99]利用细胞片技术来制备间充质干细胞片和软骨细胞片并植入缺损部位,结果发现两种细胞片均能促进生长板软骨组织的再生,同时可以有效防止骨桥形成,但两者间的差异仍需进一步比较。细胞片技术与传统支架相比的一个优势是细胞外基质具有自黏性,植入后与周围组织产生相互作用进而紧密黏合,但也存在一定缺陷,如稳定性较差、机械强度低、操作难度较大等问题,这也限制了细胞片技术的应用[100]。目前暂时没有将细胞片和基于支架策略对生长板再生治疗效果进行对比的研究,后续研究可以对这两者进行比较以提供更多的数据支持。此外,未来可以考虑将细胞片技术做为生长板再生治疗的一种辅助手段。
2.4.2 细胞聚合技术 除了细胞片外,有的研究使用无支架的细胞聚合技术来制备用于生长板软骨再生治疗的无支架聚合物。CHOW等[101]采用同种异体兔肋软骨,通过细胞聚合来构建无支架的生物软骨颗粒并将其植入生长板缺损部位,结果显示生物软骨颗粒在宿主体内持续生长并表现出与自然生长板相似的特征,在第16周,缺损部位性有早期生长板闭合的迹象,此时空白对照组已经发生闭合;而且在患肢短缩方面,相比对照组,植入组的改善程度达(41.1±7.2)%,结果表明生物软骨颗粒能防止骨桥形成或生长板过早闭合并改善肢体纵向生长能力,并具有良好的生物相容性。该技术的局限性是生物软骨颗粒的体外增殖能力在14 d达到峰值,这可能会影响生物软骨颗粒移植后的生长潜力。LEE等[102]提取兔肋软骨生长板静息区细胞,利用三维软骨细胞球团培养技术制造具有正常生长板细胞学特征的组织工程移植物,三维球团的形式能够保留软骨细胞的分化表型,同时合成和沉积软骨基质,取0.03 g(湿质量)的静息区软骨足以合成组织工程移植物,将其植入下肢生长板缺损部位后可以恢复直径3 mm缺陷的生长潜力,且移植后7周未发生明显的免疫排斥反应。该技术的优势是不会对供体部位的生长潜力和机械性能造成严重影响。YOSHIDA等[103]利用滑膜来源间充质干细胞制备一种无支架组织工程构建物,滑膜间充质干细胞及其细胞外基质形成的复合物通过活跃的组织收缩形成三维结构,该组织工程构建植入后在缺损处形成增殖性软骨细胞和前肥大软骨细胞且细胞呈生长板状柱状排列,同时能预防损伤部位的骨桥形成;此外,实验还提示,局部信号梯度可能对生长板软骨再生效果产生影响。未来的研究方向可能是模拟正常生长板的发育过程,或通过调节生长板内部的信号梯度来控制软骨细胞的增殖、分化和肥大,从而促进生长板软骨再生。然而,细胞聚合技术要实现更高精度地模拟原生组织还存在一定的挑战,例如如何控制聚合物的尺寸以及机械强度以适应原生组织的环境[104]。

| [1] NEWTON PT, LI L, ZHOU B, et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019:567(7747):234-238. [2] SAMSA WE, ZHOU X, ZHOU G. Signaling pathways regulating cartilage growth plate formation and activity. Semin Cell Dev Biol. 2017;62:3-15. [3] KAZEMI M, WILLIAMS JL. Properties of Cartilage-Subchondral Bone Junctions: A Narrative Review with Specific Focus on the Growth Plate. Cartilage. 2021:13(2_suppl):16S-33S. [4] QIAO Z, LIAN M, HAN Y, et al. Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials. 2021;266:120385. [5] SHAW N, ERICKSON C, BRYANT SJ, et al. Regenerative Medicine Approaches for the Treatment of Pediatric Physeal Injuries. Tissue Eng Part B Rev. 2018;24(2):85-97. [6] DUA K, ABZUG JM, SESKO BAUER A, et al. Pediatric Distal Radius Fractures. Instr Course Lect. 2017;66:447-460. [7] GAUGER EM, CASNOVSKY LL, GAUGER EJ, et al. Acquired Upper Extremity Growth Arrest. Orthopedics. 2017;40(1):e95-e103. [8] KARLIKOWSKI M, SUŁKO J. Physeal fractures of the lower leg in children and adolescents: Therapeutic results, pitfalls and suggested management protocol - based on the experience of the authors and contemporary literature. Adv Med Sci. 2018;63(1):107-111. [9] ZHOU FH, FOSTER BK, SANDER G, et al. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 2004;35(6): 1307-1315. [10] CHUNG R, COOL JC, SCHERER MA, et al. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 2006;80(6):1272-1280. [11] XIAN CJ, ZHOU FH, MCCARTY RC, et al. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 2004; 22(2):417-426. [12] BEAMER B, HETTRICH C, LANE J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS J. 2010;6(1):85-94. [13] SU YW, WONG DSK, FAN J, et al. Enhanced BMP signalling causes growth plate cartilage dysrepair in rats. Bone. 2021:145:115874. [14] SU YW, CHUNG R, RUAN CS, et al. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the Bony Repair of Injured Growth Plate Cartilage and Bone in Rats. J Bone Miner Res. 2016;31(6):1258-1274. [15] NGUYEN JC, MARKHARDT BK, MERROW AC, et al. Imaging of Pediatric Growth Plate Disturbances. RadiograpRadiographics. 2017;37(6):1791-1812. [16] FOX DB. Physeal Injuries and Angular Limb Deformities. Vet Clin North Am Small Anim Pract. 2021;51(2):305-322. [17] WANG X, LI Z, WANG C, et al. Enlightenment of Growth Plate Regeneration Based on Cartilage Repair Theory: A Review. Front Bioeng Biotechnol. 2021;9:654087. [18] FOSTER BK, HANSEN AL, GIBSON GJ, et al. Reimplantation of growth plate chondrocytes into growth plate defects in sheep. J Orthop Res. 1990;8(4):555-564. [19] PARK JS, AHN JI, OH DI. Chondrocyte allograft transplantation for damaged growth plate reconstruction. Yonsei Meical J. 1994;35(4):378-387. [20] JIANG T, KAI D, LIU S, et al. Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway. Biomaterials. 2018;178:281-292. [21] GAO XL, CAO MG, AI GG, et al. Mir-98 reduces the expression of HMGA2 and promotes osteogenic differentiation of mesenchymal stem cells. Eur Rev Med Pharmacol Sci. 2018;22(11):3311-3317. [22] DIXIT M, POUDEL SB, YAKAR S. Effects of GH/IGF axis on bone and cartilage. Mol Cell Endocrinol. 2021;519:111052. [23] SUNDARARAJ SKC, CIEPLY RD, GUPTA G, et al. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J Tissue Eng Regen Med. 2015;9(12):E202-209. [24] LEE S, CHOI J, MOHANTY J, et al. Structures of β-klotho reveal a ’zip code’-like mechanism for endocrine FGF signalling. Nature. 2018;553(7689):501-505. [25] NASRABADI D, REZAEIANI S, ESLAMINEJAD MB, et al. Improved Protocol for Chondrogenic Differentiation of Bone Marrow Derived Mesenchymal Stem Cells -Effect of PTHrP and FGF-2 on TGFβ1/BMP2-Induced Chondrocytes Hypertrophy. Stem Cell Rev Rep. 2018;14(5):755-766. [26] COLEMAN RM, SCHWARTZ Z, BOYAN BD, et al. The therapeutic effect of bone marrow-derived stem cell implantation after epiphyseal plate injury is abrogated by chondrogenic predifferentiation. Tissue Eng Part A. 2013:19(3-4):475-483. [27] CHEN MJ, WHITELEY JP, PLEASE CP, et al. Inducing chondrogenesis in MSC/chondrocyte co-cultures using exogenous TGF-β: a mathematical model. J Theor Biol. 2018:439:1-13. [28] MCCARTY RC, XIAN CJ, GRONTHOS S, et al. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J. 2010;4:204-210. [29] STAGER MA, ERICKSON CB, PAYNE KA, et al. Fabrication of Size-Controlled and Emulsion-Free Chitosan-Genipin Microgels for Tissue Engineering Applications. J Vis Exp. 2022; (182). doi: 10.3791/63857. [30] FURUMATSU T, TSUDA M, TANIGUCHI N, et al. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005; 280(9):8343-8350. [31] GU R, LIU H, ZHU Y, et al. Is extracellular matrix (ECM) a promising scaffold biomaterial for bone repair? Histol Histopathol. 2021;36(12):1219-1234. [32] JIA L, ZHANG P, CI Z, et al. Acellular cartilage matrix biomimetic scaffold with immediate enrichment of autologous bone marrow mononuclear cells to repair articular cartilage defects. Mater Today Bio. 2022;15:100310. [33] LI W, XU R, HUANG J, et al. Treatment of rabbit growth plate injuries with oriented ECM scaffold and autologous BMSCs. Sci Rep. 2017;7:44140. [34] ZHANG X, CHEN X, HONG H, et al. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2021;10:15-31. [35] URIST MR. Bone: formation by autoinduction. Science (New York, N.Y.). 1965;150(3698): 893-899. [36] JIN X, LUO Z, WANG J. Treatment of rabbit growth plate injuries with an autologous tissue-engineered composite. An experimental study. Cells, Tissues, Organs. 2006;183(2):62-67. [37] BADYLAK SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007; 28(25):3587-3593. [38] MIKHAILOV OV. Gelatin as It Is: History and Modernity. Int J Mol Sci. 2023;24(4):3583. [39] GROGAN SP, CHEN X, SOVANI S, et al. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue Eng Part A. 2014;20(1-2):264-274. [40] EBHODAGHE SO. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J Biomater Sci Polym Ed. 2022;33(12):1595-1622. [41] SALATI MA, KHAZAI J, TAHMURI AM, et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers (Basel). 2020;12(5):1150. [42] CHEN F, HUI JHP, CHAN WK, et al. Cultured mesenchymal stem cell transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2003;23(4):425-429. [43] BEEN S, CHOI J, CHO H, et al. Preparation and characterization of a soluble eggshell membrane/agarose composite scaffold with possible applications in cartilage regeneration. J Tissue Eng Regen Med. 2021;15(4):375-387. [44] DEEPTHI S, VENKATESAN J, KIM SK, et al. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules. Int J Biol Macromol. 2016;93(Pt B):1338-1353. [45] SUKPAITA T, CHIRACHANCHAI S, CHANAMUANGKON T, et al. Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells. 2022;11(20):3217. [46] LI L, HUI JHP, GOH JCH, et al. Chitin as a scaffold for mesenchymal stem cells transfers in the treatment of partial growth arrest. J Pediatr Orthop. 2004;24(2):205-210. [47] PLANKA L, SRNEC R, RAUSER P, et al. Nanotechnology and mesenchymal stem cells with chondrocytes in prevention of partial growth plate arrest in pigs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156(2):128-134. [48] ERICKSON C, STAGER M, RIEDERER M, et al. Emulsion-free chitosan-genipin microgels for growth plate cartilage regeneration. J Biomater Appl. 2021;36(2):289-296. [49] MAITY C, DAS N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top Curr Chem (Cham). 2021;380(1):3. [50] RASTOGI P, KANDASUBRAMANIAN B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):042001. [51] NISHIMORI S, WEIN MN, KRONENBERG HM. PTHrP targets salt-inducible kinases, HDAC4 and HDAC5, to repress chondrocyte hypertrophy in the growth plate. Bone. 2021;142:115709. [52] MIZUHASHI K, ONO W, MATSUSHITA Y, et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563(7730): 254-258. [53] OHBA S. Hedgehog Signaling in Skeletal Development: Roles of Indian Hedgehog and the Mode of Its Action. Int J Mol Sci. 2020;21(18):6665. [54] ERICKSON AG, LAUGHLIN TD, ROMEREIM SM, et al. A Tunable, Three-Dimensional In Vitro Culture Model of Growth Plate Cartilage Using Alginate Hydrogel Scaffolds. Tissue Eng Part A. 2018;24(1-2):94-105. [55] STURTIVANT A, CALLANAN A. The use of antifreeze proteins to modify pore structure in directionally frozen alginate sponges for cartilage tissue engineering. Biomed Phys Eng Express. 2020;6(5):055016. [56] FREEMAN FE, KELLY DJ. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci Rep. 2017;7(1):17042. [57] WEI L, TAN J, LI L, et al. Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. Int J Mol Sci. 2022;23(3):1249. [58] LIN H, YIN C, MO A, et al. Applications of Hydrogel with Special Physical Properties in Bone and Cartilage Regeneration. Materials (Basel, Switzerland). 2021;14(1):235. [59] YU Y, RODRIGUEZ-FONTAN F, ECKSTEIN K, et al. Rabbit Model of Physeal Injury for the Evaluation of Regenerative Medicine Approaches. Tissue Eng Part C Methods. 2019;25(12):701-710. [60] STAGER MA, THOMAS SM, ROTELLO-KURI N, et al. Polyelectrolyte Complex Hydrogels with Controlled Mechanics Affect Mesenchymal Stem Cell Differentiation Relevant to Growth Plate Injuries. Macromol Biosci. 2022;22(9):e2200126. [61] ERICKSON CB, NEWSOM JP, FLETCHER NA, et al. Anti-VEGF antibody delivered locally reduces bony bar formation following physeal injury in rats. J Orthop Res. 2021;39(8): 1658-1668. [62] ERICKSON CB, NEWSOM JP, FLETCHER NA, et al. In vivo degradation rate of alginate-chitosan hydrogels influences tissue repair following physeal injury. J Biomed Mater Res B Appl Biomater. 2020;108(6):2484-2494. [63] YUAN Z, WEI P, HUANG Y, et al. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85:294-309. [64] RAGHAV PK, MANN Z, AHLAWAT S, et al. Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine. Eur J Pharmacol. 2022;918:174657. [65] CLARK A, HILT JZ, MILBRANDT TA, et al. Treating Proximal Tibial Growth Plate Injuries Using Poly(Lactic-co-Glycolic Acid) Scaffolds. Biores Open Access. 2015;4(1):65-74. [66] COSTANTINO MD, SCHUSTER A, HELMHOLZ H, et al. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020;101:598-608. [67] WITTE F, KAESE V, HAFERKAMP H, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557-3563. [68] SONG MH, YOO WJ, CHO TJ, et al. In Vivo Response of Growth Plate to Biodegradable Mg-Ca-Zn Alloys Depending on the Surface Modification. Int J Mol Sci. 2019;20(15):3761. [69] YAZDIMAMAGHANI M, RAZAVI M, VASHAEE D, et al. Porous magnesium-based scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;71:1253-1266. [70] KIM YH, DAWSON JI, OREFFO ROC, et al. Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration. Bioengineering (Basel, Switzerland). 2022;9(7):332. [71] SHIN J, KANG EH, CHOI S, et al. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater Sci Eng. 2021;7(9):4230-4243. [72] LI S, MA F, PANG X, et al. Synthesis of chondroitin sulfate magnesium for osteoarthritis treatment. Carbohydr Polym. 2019;212:387-394. [73] GUAN P, LIU C, XIE D, et al. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact Mater. 2022;10:145-158. [74] GUAN P, JI Y, KANG X, et al. Biodegradable Dual-Cross-Linked Hydrogels with Stem Cell Differentiation Regulatory Properties Promote Growth Plate Injury Repair via Controllable Three-Dimensional Mechanics and a Cartilage-like Extracellular Matrix. ACS Appl Mater Interfaces. 2023. doi: 10.1021/acsami.2c20722. [75] CAMPBELL TM, DILWORTH FJ, ALLAN DS, et al. The Hunt Is On! In Pursuit of the Ideal Stem Cell Population for Cartilage Regeneration. Front Bioeng Biotechnol. 2022;10:866148. [76] WANG J, WANG Y, SUN X, et al. Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47(1):1710-1721. [77] ECKSTEIN KN, THOMAS SM, SCOTT AK, et al. The heterogeneous mechanical properties of adolescent growth plate cartilage: A study in rabbit. J Mech Behav Biomed Mater. 2022;128:105102. [78] SERGERIE K, LACOURSIÈRE MO, LÉVESQUE M, et al. Mechanical properties of the porcine growth plate and its three zones from unconfined compression tests. J Biomech. 2009;42(4):510-516. [79] CARTER DR, ORR TE, FYHRIE DP, et al. Influences of mechanical stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res. 1987;(219):237-250. [80] VENDRA BB, ROAN E, WILLIAMS JL. Chondron curvature mapping in growth plate cartilage under compressive loading. J Mech Behav Biomed Mater. 2018;84:168-177. [81] GAO J, WILLIAMS JL, ROAN E. Multiscale modeling of growth plate cartilage mechanobiology. Biomechanics and Modeling in Mechanobiology. 2017;16(2):667-679. [82] HEINRICH MA, LIU W, JIMENEZ A, et al. 3D Bioprinting: from Benches to Translational Applications. Small (Weinheim an Der Bergstrasse, Germany). 2019;15(23):e1805510. [83] Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact Mater. 2018;3(2): 144-156. [84] HUGHES AM, KOLB AD, SHUPP AB, et al. Printing the Pathway Forward in Bone Metastatic Cancer Research: Applications of 3D Engineered Models and Bioprinted Scaffolds to Recapitulate the Bone-Tumor Niche. Cancers. 2021;13(3):507. [85] YU Y, FISCHENICH KM, SCHOONRAAD SA, et al. A 3D printed mimetic composite for the treatment of growth plate injuries in a rabbit model. NPJ Regen med. 2022:7(1):60. [86] ZHANG L, YANG G, JOHNSON BN, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33. [87] NI J, LING H, ZHANG S, et al. Three-dimensional printing of metals for biomedical applications. Mater Today Bio. 2019;3:100024. [88] WANG Y, CUI H, ESWORTHY T, et al. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical Devices. Adv Mater. 2022;34(20):e2109198. [89] LI W, XU R, HUANG J, et al. Treatment of rabbit growth plate injuries with oriented ECM scaffold and autologous BMSCs. Sci Rep. 2017;7:44140. [90] XU T, BINDER KW, ALBANNA MZ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. [91] LEE JK, LINK JM, HU JCY, et al. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb Perspect Med. 2017;7(11):a025668. [92] SUN K, TAO C, WANG DA. Scaffold-free approaches for the fabrication of engineered articular cartilage tissue. Biomed Mater. 2022:17(2). doi: 10.1088/1748-605X/ac51b9. [93] DE PIERI A, ROCHEV Y, ZEUGOLIS DI. Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast. NPJ Regen Med. 2021;6(1):18. [94] HARAGUCHI Y, SHIMIZU T, SASAGAWA T, et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012:7(5):850-858. [95] LI M, MA J, GAO Y, et al. Cell sheet technology: a promising strategy in regenerative medicine. Cytotherapy. 2019;21(1):3-16. [96] KAWECKI F, CLAFSHENKEL WP, FORTIN M, et al. Biomimetic Tissue-Engineered Bone Substitutes for Maxillofacial and Craniofacial Repair: The Potential of Cell Sheet Technologies. Adv Healthc Mater. 2018;7(6):e1700919. [97] DING J, BAO S, QIAN W, et al. Subcutaneous Regeneration of Engineered Cartilage: A Comparison of Cell Sheets and Chondrocyte-Scaffold Constructs in a Porcine Model. Plast Reconstr Surg. 2021;147(3):625-632. [98] SATO M, YAMATO M, MITANI G, et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019;4:4. [99] GÜLTEKIN A, AĞIRDIL Y, ÖNCEL DUMAN B, et al. Comparison of mesenchymal stem cell sheets and chondrocyte sheets in a rabbit growth plate injury model. Turk J Med Sci. 2020;50(4):1082-1096. [100] KONDO M, KAMEISHI S, GRAINGER DW, et al. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerg Top Life Sci. 2020;4(6):677-689. [101] CHOW SKH, LEE KM, QIN L, et al. Restoration of longitudinal growth by bioengineered cartilage pellet in physeal injury is not affected by low intensity pulsed ultrasound. J Biomed Mater Res B Appl Biomater. 2011;99(1):36-44. [102] LEE KM, CHENG ASL, CHEUNG WH, et al. Bioengineering and characterization of physeal transplant with physeal reconstruction potential. Tissue Eng. 2003;9(4):703-711. [103] YOSHIDA K, HIGUCHI C, NAKURA A, et al. Treatment of partial growth arrest using an in vitro-generated scaffold-free tissue-engineered construct derived from rabbit synovial mesenchymal stem cells. J Pediatr Orthop. 2012;32(3):314-321. [104] COOPER SM, RAINBOW RS. The Developing Field of Scaffold-Free Tissue Engineering for Articular Cartilage Repair. Tissue Eng Part B Rev. 2022;28(5):995-1006. |
| [1] | 杨玉芳, 杨芷姗, 段棉棉, 刘毅恒, 唐正龙, 王 宇. 促红细胞生成素在骨组织工程中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1443-1449. |
| [2] | 陈凯佳, 刘景云, 曹 宁, 孙建波, 周 燕, 梅建国, 任 强. 组织工程技术在股骨头坏死治疗中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1450-1456. |
| [3] | 梅静怡, 刘 江, 肖 聪, 刘 鹏, 周浩浩, 林展翼. 组织工程血管构建过程中平滑肌细胞增殖变化及代谢模式[J]. 中国组织工程研究, 2024, 28(7): 1043-1049. |
| [4] | 王姗姗, 舒 晴, 田 峻. 物理因子促进干细胞的成骨分化[J]. 中国组织工程研究, 2024, 28(7): 1083-1090. |
| [5] | 刘瀚峰, 王晶晶, 余云生. 人造外泌体治疗心肌梗死:应用现状及前景[J]. 中国组织工程研究, 2024, 28(7): 1118-1123. |
| [6] | 沈子青, 夏 天, 单一波, 朱睿君, 万昊鑫, 丁 浩, 潘 枢, 赵 军. 负载外泌体水凝胶修饰3D打印支架构建血管化的气道替代物[J]. 中国组织工程研究, 2024, 28(5): 697-705. |
| [7] | 王建春, 杨树青, 苏 欣, 王宏远. 不同含量B2O3对生物活性玻璃支架力学性能与生物活性的影响[J]. 中国组织工程研究, 2024, 28(5): 712-716. |
| [8] | 张艺海, 商 鹏, 马奔原, 侯光辉, 崔伦旭, 宋万振, 齐德瑄, 刘艳成. 径向梯度三周期极小曲面骨小梁支架结构设计与力学性能分析[J]. 中国组织工程研究, 2024, 28(5): 741-746. |
| [9] | 朱礼威, 王江玥, 白 丁. 纳米复合甲基丙烯酰明胶水凝胶在不同骨缺损环境中应用的价值[J]. 中国组织工程研究, 2024, 28(5): 753-758. |
| [10] | 陈小芳, 郑国爽, 李茂源, 于炜婷. 可注射海藻酸钠水凝胶的制备及应用[J]. 中国组织工程研究, 2024, 28(5): 789-794. |
| [11] | 王嘉旎, 陈俊宇. 金属离子促血管生成机制及在骨组织工程中的应用[J]. 中国组织工程研究, 2024, 28(5): 804-812. |
| [12] | 杨雨晴, 陈志宇. 早期短暂M1巨噬细胞在骨组织工程中的作用及应用[J]. 中国组织工程研究, 2024, 28(4): 594-601. |
| [13] | 孔祥宇, 王 兴, 裴志伟, 常家乐, 李斯琴, 郝 廷, 何万雄, 张葆鑫, 贾燕飞. 生物支架材料及打印技术修复骨缺损[J]. 中国组织工程研究, 2024, 28(3): 479-485. |
| [14] | 徐 静, 吕慧欣, 鲍 鑫, 张 逸, 王一涵, 周延民. 近红外光响应水凝胶在组织工程领域的应用[J]. 中国组织工程研究, 2024, 28(3): 486-492. |
| [15] | 谷明西, 王常成, 田丰德, 安 宁, 郝瑞胡, 郭 林. 丝素蛋白/明胶/壳聚糖三维多孔软骨组织支架的制备及体外评价[J]. 中国组织工程研究, 2024, 28(3): 366-372. |
生长板是儿童骨骼中最脆弱的区域,骨折、感染、肿瘤或医源性损伤等均可导致生长板损伤,约30%的儿童骨折伴有生长板损伤[6-8]。目前通过动物模型发现生长板损伤后会经历4个时期,即炎症期、成纤维期、成骨期和重塑期[9]。炎症期出现在生长板损伤后3 d内,此时大量炎症细胞在损伤后约8 h内快速浸润损伤部位[9],参与调节生长板骨修复的下游成软骨和成骨事件[10];在成纤维期,大量具有成骨和成软骨能力的间充质干细胞聚集在损伤部位并对后续过程产生影响[11];当生长板损伤修复进入成骨期,损伤部位开始出现骨小梁并伴有新生血管的侵入,此时骨桥逐渐形成[12];进入重塑期,损伤部位出现大量的骨小梁和骨髓组织[13],破骨细胞分化与聚集以促进骨重塑的过程[14]。由于生长板再生能力差及未成熟骨骼具有动态特性[15],因此生长板损伤后缺损部位易发生骨组织修复,形成“骨桥”,并导致生长阻滞、成角或旋转畸形,造成严重的远期并发症,给儿童的身心健康带来巨大影响[16]。目前临床治疗方法主要为骨桥切除术结合相应的高分子材料填充,以防止骨桥复发并促进周围未受损伤的生长板组织恢复骨骼纵向生长能力,尽管如此,这种治疗方法的成功率仍然非常低,后期多需要接受截骨矫形或肢体延长等手术[9],由于手术预后的不可预测性和不合适填充材料导致的较高骨桥再形成率,迫切需要一种新的治疗方式来治疗生长板损伤。近年来,组织工程被认为是一种潜在的生长板再生治疗方式。组织工程技术是将种子细胞与生物活性因子结合并植入支架中,形成具有组织再生功能的生物材料,随后植入缺损部位,通过修复或替代损伤的组织来实现组织损伤后的再生。采用组织工程技术构建具有再生功能的生长板损伤修复材料,来防止骨桥形成并促进组织再生具有重大意义,具备治疗儿童生长板损伤的潜力。目前针对生长板再生治疗的研究大多仍采用基于支架的再生治疗策略,然而,随着传统支架局限性带来的问题逐渐显露,对于后续的研究方向也出现了分歧。因此,该综述回顾了在生长板软骨损伤的背景下组织工程支架的研究进展,总结基于支架的再生治疗策略和无支架策略之间存在的优势和不足,以期为后续应用于生长板软骨再生的支架设计提供新思路和新策略。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
1.1.1 检索人及检索时间 由第一作者在2022年12月至2023年2月进行检索。
1.1.2 检索文献时限 查阅1990-2023年期间收录的关于生长板再生治疗研究进展情况的相关文章。
1.1.3 检索数据库 PubMed、Wiley、Elsevier数据库。
1.1.4 检索词 英文检索词为“growth plate injury,regeneration,tissue engineering,scaffold,scaffold-free,biomimetic”。
1.1.5 检索文献类型 综述、基础及临床研究。
1.1.6 手工检索情况 无。
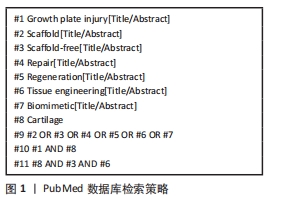
1.1.7 检索策略 以PubMed数据库为例,检索策略见图1。
1.2 入选标准
1.2.1 纳入标准 基于支架策略和无支架策略在生长板再生治疗中所取得效果相关的研究;同一领域中论点、论据可靠的文献。
1.2.2 排除标准 与课题内容不一致的文献;内容较陈旧、结论重复、可信度较低的文章;与文章相关性不强、借鉴价值不高的文献。
1.3 数据的提取 研究内容由作者独立提取并通过研究筛选,重点关注组织工程支架在生长板再生治疗中所取得的新进展。
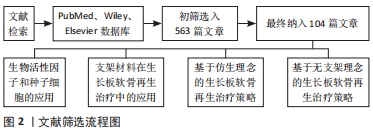
1.4 质量评估 应用计算机初检得到563篇文献,通过阅读文题和摘要进行初步筛选,排除与文章主题不相关的文献,根据纳入标准和排除标准最后纳入104篇文献进行综述分析,见图2。
 3.2 作者综述区别于他人他篇的特点 先前大多数研究是基于支架策略而进行的,然而随着组织工程技术和再生医学的发展,基于支架的生长板再生治疗策略也发生转变,并衍生出仿生支架策略和无支架策略。因此,文章对支架材料的最新进展进行汇总,包括材料的优缺点以及最新的研究如何去利用材料的优势或弥补劣势。文章也提出目前的研究方向存在的分歧(即对于下一步研究应该采用仿生策略还是无支架策略进行生长板再生组织工程的设计),并总结仿生策略和无支架策略在生长板再生治疗中所取得成果以及各自优劣。仿生支架策略是通过模拟生长板独特的组织结构最大程度上还原每个区域的细胞组成、生物信号和独特力学性能,进而构建能促进组织再生的仿生微环境,因此,仿生支架的设计则是尽可能地从成分、结构和力学性能上模拟原生生长板,虽然取得一定的成效,但仍存在再生效果不稳定的问题。而无支架策略认为支架的局限性会对再生治疗产生不利的影响,因此,无支架构建物的设计则是尽可能地依赖于细胞自身产生细胞外基质并维持的能力,不干扰细胞-细胞间的信号、不引入外源性物质,但存在稳定性较差、机械强度低、操作难度较大等问题。
3.2 作者综述区别于他人他篇的特点 先前大多数研究是基于支架策略而进行的,然而随着组织工程技术和再生医学的发展,基于支架的生长板再生治疗策略也发生转变,并衍生出仿生支架策略和无支架策略。因此,文章对支架材料的最新进展进行汇总,包括材料的优缺点以及最新的研究如何去利用材料的优势或弥补劣势。文章也提出目前的研究方向存在的分歧(即对于下一步研究应该采用仿生策略还是无支架策略进行生长板再生组织工程的设计),并总结仿生策略和无支架策略在生长板再生治疗中所取得成果以及各自优劣。仿生支架策略是通过模拟生长板独特的组织结构最大程度上还原每个区域的细胞组成、生物信号和独特力学性能,进而构建能促进组织再生的仿生微环境,因此,仿生支架的设计则是尽可能地从成分、结构和力学性能上模拟原生生长板,虽然取得一定的成效,但仍存在再生效果不稳定的问题。而无支架策略认为支架的局限性会对再生治疗产生不利的影响,因此,无支架构建物的设计则是尽可能地依赖于细胞自身产生细胞外基质并维持的能力,不干扰细胞-细胞间的信号、不引入外源性物质,但存在稳定性较差、机械强度低、操作难度较大等问题。3.3 综述的局限性 涉及支架在生长板再生治疗效果方面相关进展的文献及相关实验数据的数量还不够多,特别是在无支架策略和仿生支架策略两个方面的实验研究较少,无法综述形成确信度较高的未来研究思路。
3.4 文章的重要意义 文章认为仿生策略和无支架策略的出发点不同且均存在各自的优缺点,但它们对于生长板软骨再生均能产生积极的作用。因此,后续的研究不论是采取仿生策略或无支架策略,都将聚焦于对现有技术的不断优化,以达到最终实现生长板软骨损伤有效治疗的目标。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
生长板:又称骺板,是儿童未成熟长骨末端控制骨骼纵向生长的软骨区域,同时也是儿童骨骼中最脆弱的区域。由于生长板软骨再生能力差以及未成熟骨骼具有动态特性,一旦发生损伤,生长板损伤后缺损部位易发生骨组织修复,进而形成“骨桥”,并导致生长阻滞、成角或旋转畸形,造成严重远期并发症。三维支架:通常用于组织工程领域的药物输送、细胞移植以及细胞行为和材料研究。目前组织工程学的三维支架通常是采用具有生物相容性和可生物降解的材料,目的是构建一个合适的微环境,为最佳的细胞生长和功能提供相应的支持。
随着组织工程技术合和再生医学的发展,基于支架的生长板板再生治疗策略也发生转变,并衍生出仿生支架策略和无支架策略。仿生支架通过模拟每个区域的细胞组成、生物信号、和独特力学性能,以构建适合再生的微环境,而无支架策略则依赖于细胞自身细胞产生和维持自身细胞外基质的能力来尝试还原体内组织发育的微环境。无论是组织工程支架或者是无支架,都具有各自的优势和不足,且两者之间并非互相替代的关系。未来的方向可以采取并行发展,甚至是尝试进一步将两者结合起来,达到克服各自缺点和发挥优点的作用,推动生长板再生治疗向新的层次前进。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||