中国组织工程研究 ›› 2021, Vol. 25 ›› Issue (13): 2011-2017.doi: 10.3969/j.issn.2095-4344.3512
• 脂肪干细胞 adipose-derived stem cells • 上一篇 下一篇
脂肪间充质干细胞治疗系统性硬化症模型小鼠的机制
张晶莹1,孙晓林2,耿立霞1
- 1内蒙古科技大学包头医学院第一附属医院重症医学科,内蒙古自治区包头市 014010;2包头医学院第一附属医院风湿免疫科(包头医学院风湿免疫研究所),内蒙古自治区自体免疫学重点实验室,内蒙古自治区包头市 014010
Therapeutic mechanism of adipose mesenchymal stem cells in mice with systemic sclerosis
Zhang Jingying1, Sun Xiaolin2, Geng Lixia1
- 1Department of Intensive Medicine, First Affiliated Hospital of Baotou Medical College, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia Autonomous Region, China; 2Key Autoimmunity Laboratory of Inner Mongolia, Institute of Rheumatology, Department of Rheumatology, First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia Autonomous Region, China
摘要:
 文题释义:
文题释义:
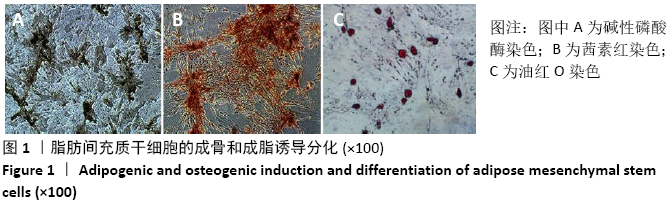
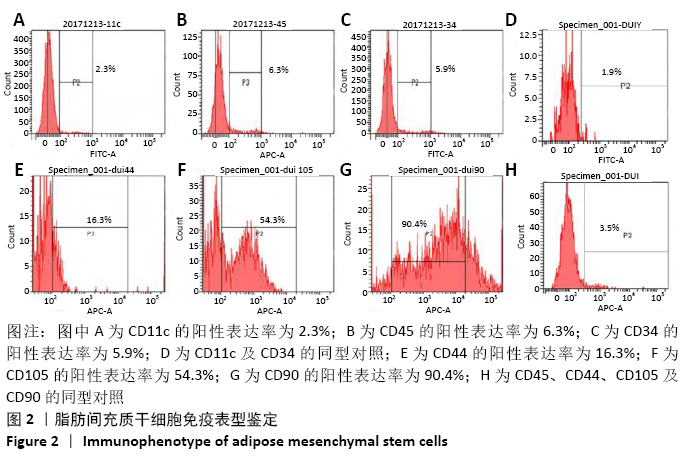
脂肪间充质干细胞:间充质干细胞是来源于发育早期中胚层的一群具有多向分化潜能、低免疫原性、可自我复制的多能干细胞,已有研究证实间充质干细胞可以促进皮肤创面的修复。脂肪间充质干细胞与其他干细胞相比,具有来源广泛、方便处理及免疫调节能力更强等优势。
系统性硬化症:是一种病理表现为慢性炎症浸润、组织及器官纤维增生的自身免疫性疾病,约2/3的系统性硬化症患者会累及肺部,主要表现为肺间质纤维化及肺动脉高压,其中肺动脉高压会导致患者通气功能和换气功能障碍,是系统性硬化症患者死亡的常见原因。
背景:虽然已有临床研究发现,自体脂肪间充质干细胞可以有效减轻辐射诱导的系统性硬化症患者面部的纤维化,但其作用机制尚未被深入分析。
目的:探讨脂肪间充质干细胞对博来霉素诱导的系统性硬化症模型小鼠的作用机制。
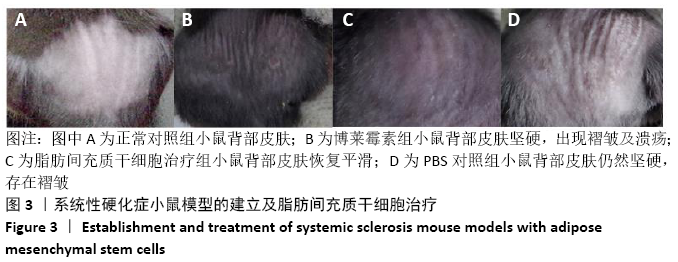
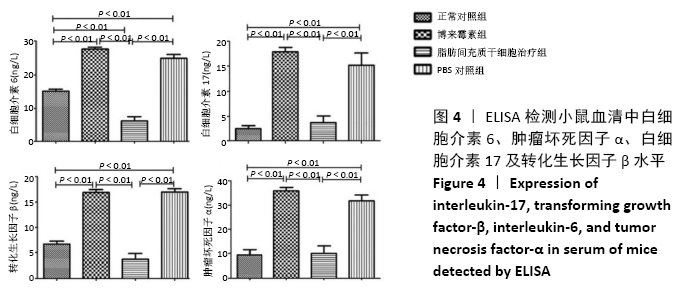
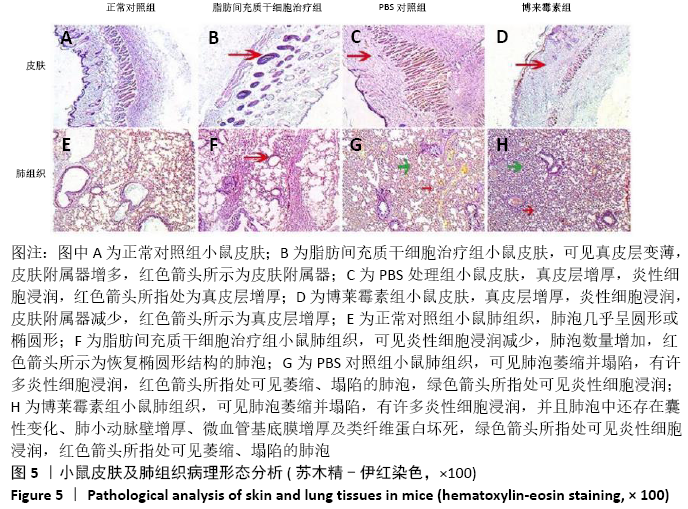
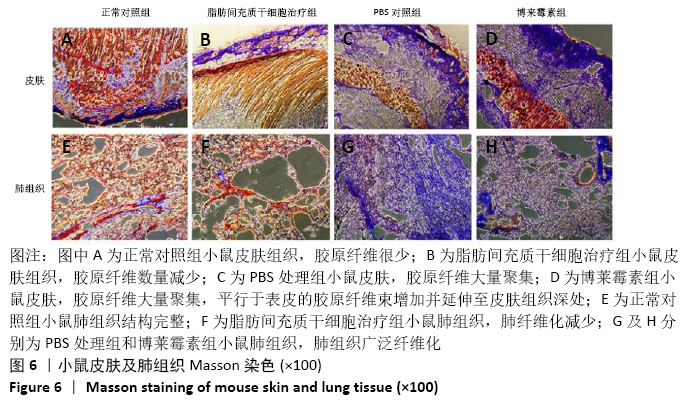
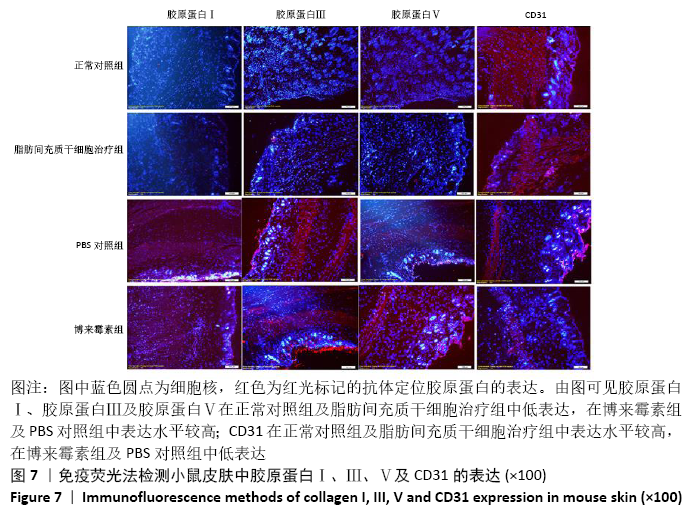
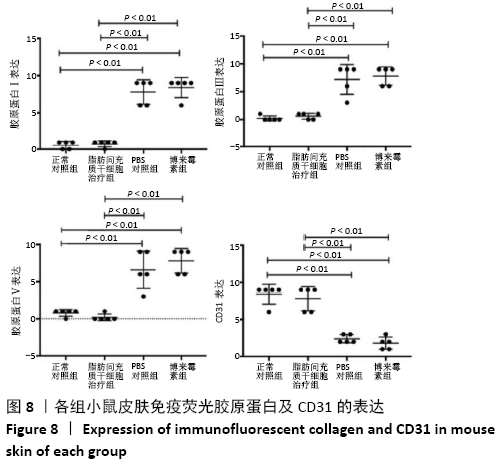
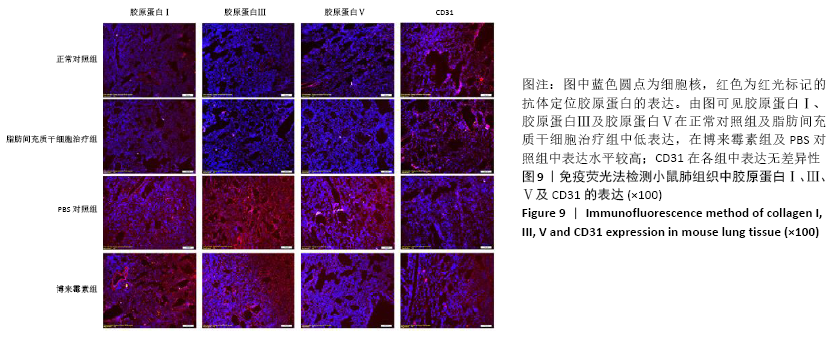
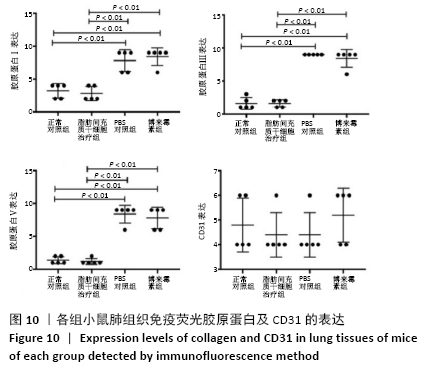
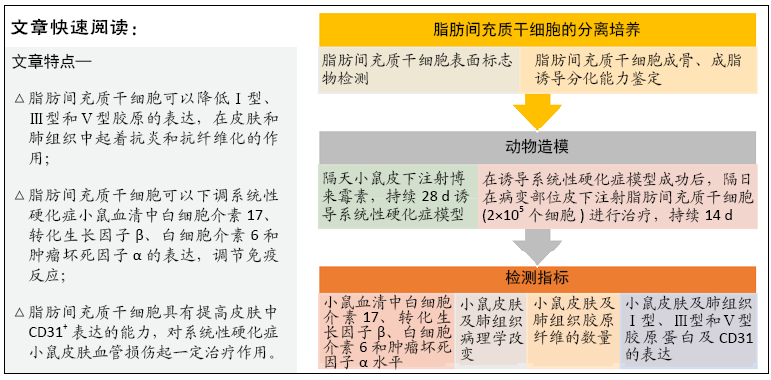
方法:取SPF级 6-8周龄C57BL/6J雌鼠40只随机分为正常对照组、脂肪间充质干细胞治疗组、博莱霉素组和PBS组,后3组每隔1 d皮下注射博莱霉素,持续28 d,建立系统性硬化症小鼠模型。造模成功后脂肪间充质干细胞组给予皮下注射脂肪间充质干细胞治疗,PBS组皮下注射PBS,均持续治疗14 d。通过ELISA法检测各组小鼠血清中白细胞介素17、转化生长因子β、白细胞介素6和肿瘤坏死因子α水平,苏木精-伊红染色和Masson染色观察系统性硬化症小鼠皮肤和肺组织病变,免疫荧光法检测小鼠皮肤和肺组织中胶原蛋白Ⅰ、Ⅲ、Ⅴ及CD31的表达水平。
结果与结论:①与博莱霉素组相比,脂肪间充质干细胞治疗组白细胞介素17、转化生长因子β、白细胞介素6和肿瘤坏死因子α的表达水平显著降低(P < 0.01);②与正常对照组相比,博莱霉素组小鼠皮肤真皮层增厚,炎症细胞浸润,皮肤附属器减少;肺泡萎缩塌陷,有许多炎性细胞浸润,肺小动脉壁增厚、微血管基底膜增厚及类纤维蛋白坏死,经脂肪间充质干细胞治疗后炎性症状有所改善;③与正常对照组相比,博莱霉素组小鼠的皮肤和肺组织中胶原纤维大量聚集,经脂肪间充质干细胞治疗后胶原纤维减少;④经脂肪间充质干细胞治疗后,博莱霉素组小鼠皮肤和肺组织中胶原蛋白Ⅰ、Ⅲ、Ⅴ表达水平降低,皮肤组织中CD31表达增多(P < 0.01);⑤结果表明,脂肪间充质干细胞可调节博莱霉素小鼠的免疫反应,具有抗纤维化、缓解炎症和减轻血管病变的作用。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: