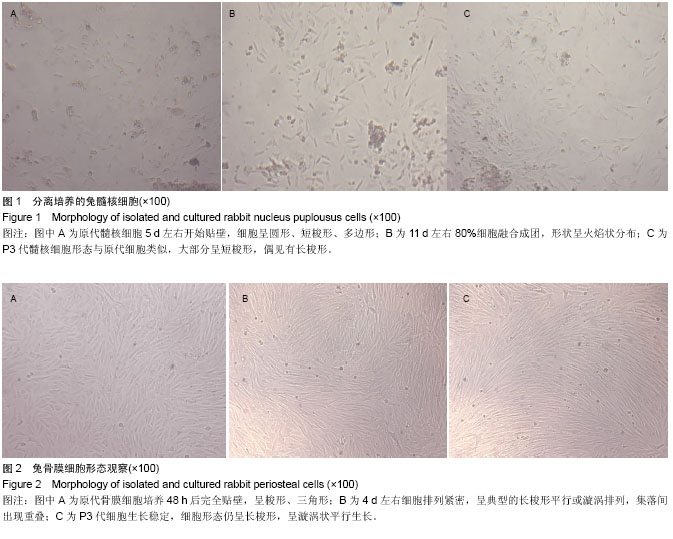
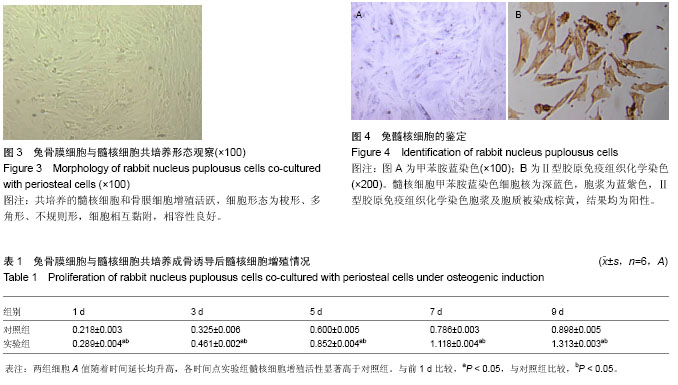
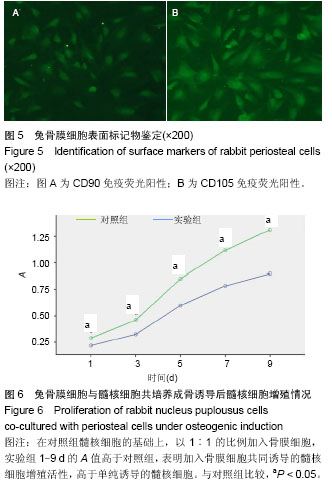
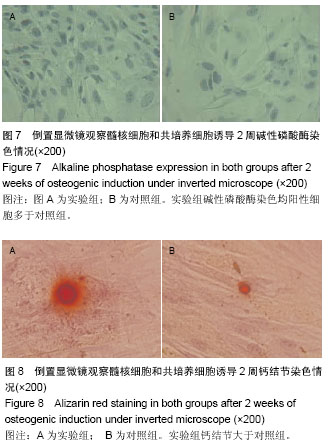
| [1] Miyazaki M, Tsumura H, Wang JC, et al. An update on bone substitutes for spinal fusion. Eur Spine J.2009;18(6 ):783-799
[2] Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in acanine model : atreatment for degenerated damaged intervertebral disc.Spine.2003;28 (23):2609- 2620.
[3] Zhang Y,Drapeau S,Howard SA,et al.Transplantion of goat bone marrow stromal cells to the degenerating intervetebral disc in a goat disc-injury model. Spine.2011;36(5):372-377.
[4] Wang YT, Wu XT, Wang F. regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration.J Orthop Sci.2010;15(6): 707-719.
[5] 陈文坚,李锋,杨晶,等.三维共培养免髓核细胞诱导骨髓间充质干细胞分化[J].中国组织工程研究与临床康复,2009,13(49): 9783-9786
[6] Choi ST, Kim JH, Kang EJ,et al.Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology(Oxford).2008; 47(12): 1775 -1779.
[7] Bobacz K, Ullrich R, Amoyo L, et al. Stimulatory effects of distinct members of the bone morphogenetic protein family on ligament fibroblasts. Ann Rheum Dis. 2006;65(2):169-177.
[8] 王哲,胡学昱,罗卓荆,等. 氟化钠对人黄韧带细胞碱性磷酸酶活性及骨钙素合成的影响[J].中华骨科杂志, 2010, 30(11):1138- 1143.
[9] Samee M, Kasugai S, Kondo H, et al. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. Pharmacol Sci. 2008; 108(1):18-31.
[10] Yu YY,Lieu S,Lu C et al. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone.2010; 47(1):65-73.
[11] van Gastel N,Torrekens S,Roberts SJ,et al.engineering vascularized bone: osteogenic and proangiogenic potential of murine periosteal cells.Stem Cells.2012;30(11):2460-2471.
[12] Arnsdorf EJ, Jones LM, Carter DR,et al. The periosteum as a cellular source for functional tissue engineering. Tissue Eng Part A 2009;9: 2637-2642.
[13] Hutmacher DW, Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng.2003;9:S45-S64.
[14] Choi YS, Noh SE, Lim SM, et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett.2008;30:593-601.
[15] Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17 Suppl 4: 452-458.
[16] Alini M, Eisenstein SM, Ito K, et al. Are animal models usefulfor studying human disc disorders/degeneration? Eur Spine J.2008;17(1):2-19.
[17] Yang Z, Huang CY, Candiotti KA, et al. Sox-9 facilitates differentiation of adipose tissue-derived stem cells into a chondrocyte-like phenotype in vitro. J Orthop Res.2011;29(8): 1291-1297.
[18] Muschik M,Schlenlzka D,Ritsil V, el al.Experimental anterior spine fusion using bovine bonemorphogenetic protein: a study in rabbits.Orthop Sci.2000;5(2):165-170
[19] 贝抗胜,孙庆文,熊英辉,等.成骨因子BMP7在骨膜细胞体外培养中的作用[J].中华显微外科杂志,2010,33(5). 384-387.
[20] 贝抗胜,吴礼杨,孙庆文,等.人骨膜细胞生物学特性的实验研究[J].中华创伤骨科杂志, 2011,13(12):1170-1174.
[21] Bei K, Du Z, Xiong Y, et al. BMP7 can promote osteogenic differentiation of human periosteal cells in vitro. Mol Biol Rep. 2012;39(9):8845-8851.
[22] McCarthy TL,Madri JA,Centrella M,et al.Stratified control of IGF-I expression by hypoxia and stress hormones in osteoblasts.Gene.2014;539(1): 141-151. |