[1] 马远征,王以朋,刘强,等.中国老年骨质疏松症诊疗指南(2018)[J].中国骨质疏松志, 2018, 24(12):1541-1567.
[2] 周隆,任兆舟,张展,等. 运动疗法对防治绝经后妇女骨质疏松症的疗效分析及对骨密度的影响[J]. 中国妇幼保健, 2020, 35(7): 1270-1273.
[3] LETAROUILLY JG,BROUX O,CLABAUT A.New insights into the epigenetics of osteoporosis.Genomic.2019; 111(4):793-798.
[4] 胡晓青,张辛,代岭辉,等.骨髓间充质干细胞成骨分化过程中Runx2的表观遗传学修饰[J].中国生物化学与分子生物学报, 2014, 30(2):150-155.
[5] CHISATI EM, CONSTANTINOU D, LAMPIAO F. Management of reduced bone mineral density in HIV:pharmacological challenges and the role of exercise. Front Physiol.2018; 7(9):1074.
[6] CHEN R, REN L, CAI Q, et al. The role of epigenetic modifications in the osteogenic differentiation of adipose-derived stem cells. Connect Tissue Res. 2019;60(6):507-520.
[7] 赵倩, 王巍, 孙野青. 植物DNA甲基化与基因表达的关联性研究进展[J]. 生物技术通报, 2016, 32(4):16-23.
[8] XU L, LIU Y, SUN Y, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017;8(1):275.
[9] 王剑,李屹,王实,等.基于骨组织Runx2、Osterix启动子甲基化探究鹿茸中药复方对去卵巢骨质疏松症大鼠的疗效机制[J/OL]. 世界科学技术-中医药现代化, 2020:1-6.
[10] WAKITANI S, YOKOI D, HIDAKA Y, et al. The differentially DNA-methylated region responsible for expression of runt-related transcription factor 2. J Vet Med Sci.2017; 79:230-237.
[11] 朱筱. 1、Runx2甲基化状态变化对血管平滑肌细胞成骨样分化影响的研究 2、Vaspin对人成骨细胞凋亡的作用机制研究[D]. 长沙:中南大学, 2012:34-50.
[12] HAGH MF, NORUZINIA M, MORTAZAVI Y, et al. Different methylation patterns of RUNX2, OSX, DLX5and BSP in osteoblastic differentiation of mesenchymal stem cells. Cell J.2015;17:71-82.
[13] RAJE MM, ASHMA R. Epigenetic regulation of BMP2 gene in osteoporosis: a DNA methylation study. Mol Biol Rep.2019;46(8):1667-1674.
[14] FU B, WANG H, WANG J, et al. Epigenetic Regulation of BMP2 by 1,25-dihydroxyvitamin D3 through DNA Methylation and Histone Modification. PLoS ONE. 2013; 8(4):e61423.
[15] 张瑞鹏. DNA去甲基化在脂肪干细胞分化与红细胞发育中的作用研究[D]. 浙江大学, 2011:33-39.
[16] 张爽, 高艳虹. sclerostin在骨代谢中的作用和机制[J].上海交通大学学报(医学版), 2015, 35(4):589-593.
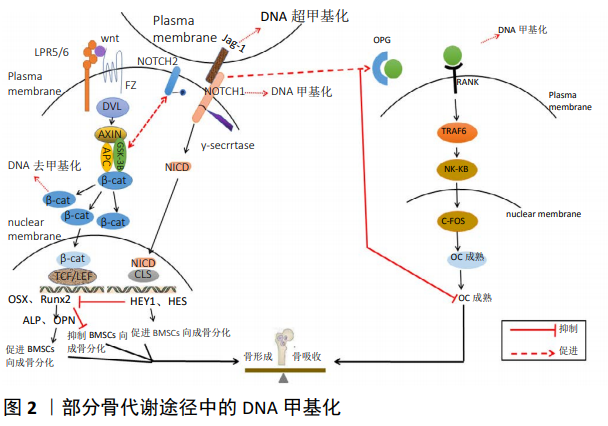
[17] 牛亚丹, 林伊荷, 张汉清, 等. DNA甲基化与骨代谢调节及骨质疏松症研究进展[J].生命科学, 2020, 32(2):162-169.
[18] 时宇博,朱福良,李立军,等. 绝经后骨质疏松症患者血浆SOST表达变化及其与基因甲基化的关系[J]. 山东医药, 2016, 56(16):35-37.
[19] YANG F, TANG W, SO S, et al. Sclerostin is a direct target of osteoblast-specific transcription factor osterix. Biochem Biophys Res Commun. 2010;400(4):684-688.
[20] 张丽君,王艺璇,胡泽兵,等. 表观遗传学调控骨骼细胞功能的研究进展[J].解放军医学院学报, 2019, 40(12):1199-1202+1206.
[21] HONG L, KATHARINA SB, ANKE D, et al. Regulatory T cell-mediated anti- inflammatory effects promote successful tissue repair in both indirect and direct manners.Front Pharmacol . 2015;6:184.
[22] 李子祺,葛鸿庆,王君鳌,等. 外周血CD4+T细胞Foxp3基因CNS2去甲基化与绝经后骨质疏松症的关系[J].实用医学杂志, 2018, 34(14):2329-2332.
[23] DAO C, LIU, SI H, et al. Different effects of Wnt/β-catenin activation and parathyroid hormone on diaphyseal and metaphyseal in the early phase of femur bone healing of mice.Clin Exp Pharmacol Physiol. 2019; 46(7):652-663.
[24] GARCÍA-IBARBIA C, DELGADO-CALLE J, CASAFONT I, et al. Contribution of genetic and epigenetic mechanisms to Wnt pathway activity in prevalent skeletal disorders. Gene.2013; 532(2):165-172.
[25] 潘乐.补肾药对BMSCs成骨与成脂分化及β-catenin、PPARγ甲基化的影响[D].陕西中医学院, 2014.
[26] WU F, JIAO J, LIU F, et al. Hypermethylation of Frizzled1 is associated with Wnt/β-catenin signaling inactivation in mesenchymal stem cells of patients with steroid-associated osteonecrosis. Exp Mol Med.2019; 51(2): 23.
[27] TARFIEI G, NORUZINIA M, SOLEIMANI M, et al. ROR2 promoter methylation change in osteoblastic differentiation of mesenchymal Stem Cells. Cell J.2011; 13(1):11-15.
[28] LEE S Y, LONG F. Notch signaling suppresses glucose metabolism in mesenchymal progenitors to restrict osteoblast differentiation. J Clin Invest. 2018;128(12):5573-5586.
[29] ZHOU Y, LI J, ZHOU K, et al. The methylation of Notch1 promoter mediates the osteogenesis differentiation in human aortic valve interstitial cells through Wnt/β‐catenin signaling.J Cell Physiol. 2019; 234(11):20366-20376.
[30] HADJI F, BOULANGER M, GUAY S, et al. Altered DNA Methylation of Long Noncoding RNA H19 in Calcific Aortic Valve Disease Promotes Mineralization by Silencing NOTCH1. Pubmed.2016;134(23):1848-1862.
[31] 傅涛, 李佳欢, 蔡逊. Notch信号通路在胃肠道肿瘤机制研究中的新进展[J]. 肿瘤学杂志, 2015, 21(10):852-855.
[32] 任磊, 代光明, 林枭, 等. 骨细胞 Wnt/β-Catenin 通过 Notch 信号促进 BMSCs 成骨分化[J]. 中国骨质疏松杂志, 2018, 24(5):600-605.
[33] ROCHETTE L, MELOUX A, RIGAL E, et al. The Role of Osteoprotegerin in Vascular Calcification and Bone Metabolism: The Basis for Developing New Therapeutics. Calcif Tissue Int. 2019;105(3):239-251.
[34] 李子怡, 李玉坤. OPG/RANK/RANKL信号通路在骨质疏松症中的研究进展和应用[J]. 中华老年骨科与康复电子杂志, 2017, 3(2): 124-128.
[35] KITAZAWA S, KITAZAWA R. Epigenetic control of mouse receptor activator of NF-κB ligand gene expression. Biochem Biophys Res Commun. 2002;293(1):126-131.
[36] DELGADO-CALLE, JESÚS, FERNÁNDEZ, et al. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics. 2012; 7(1):83-91.
[37] HUSAIN A, JEFFRIES MA. Epigenetics and Bone Remodeling.Curr Osteoporos Rep. 2017;15(5):450-458.
[38] WANG P, CAO Y, ZHAN D, et al.Influence of DNA methylation on the expression of OPG/RANKL in primary osteoporosis. Int J Med Sci. 2018; 15(13):1480-1485.
[39] 李永贤, 张顺聪, 郭丹青. Notch信号通路调控OPG/RANKL/RANK系统对骨质疏松椎体骨折影响的研究进展[J]. 中国中医骨伤科杂志, 2015, 23(11):73-77.
[40] 胡晓磐,李世昌,孙朋. 生物力学因子YAP/TAZ与骨代谢的关系[J]. 生命科学, 2019, 31(6):602-608.
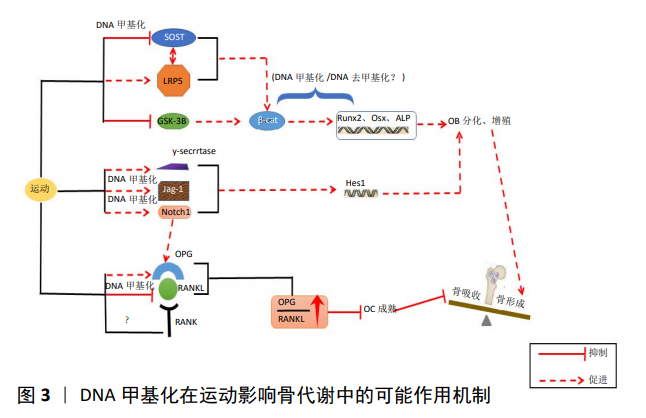
[41] 仝晓阳,张玲莉,郭健民,等. 运动对骨代谢信号通路影响的研究进展[J].中国康复理论与实践, 2016, 22(12):1425-1429.
[42] 杨念恩.不同方式运动对生长期小鼠骨合成代谢和Wnt信号通路的影响[D].上海:华东师范大学, 2014.
[43] CASE N, THOMAS J, XIE Z, et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone.2013; 52(1):454-464.
[44] RATH B, NAM J, DESCHNER J, et al. Biomechanical forces exertanabolic effects on osteoblasts by activation of SMAD 1/5/8 through type 1 BMP receptor. Biorheology. 2011; 48(1):37-48.
[45] 陈熙, 郭健 民, 元宇, 等. 不同牵张应力对成骨细胞MC3T3-E1分化及Wnt信号转导通路的影响研究[J]. 中国骨质疏松杂志, 2016, 22(1): 9-13.
[46] GAO H, ZHAI M, WANG P, et al. Low level mechanical vibration enhances osteoblastogenesis via a canonical Wnt signaling associated mechanism. Pubmed.2017;16(1):317-324.
[47] 刘晨涛, 张娟娟, 苗华, 等. 负重爬梯运动调节去卵巢大鼠OPG/Ikkα/Runx2基因表达以及对股骨结构和生物力学的影响[J]. 中国运动医学杂志, 2019, 38(4):296-304.
[48] 何标,徐波,张宪亮.运动通过提高DNA甲基化水平改善阿尔茨海默病小鼠空间学习记忆能力[J].天津体育学院学报, 2016, 31(4): 333-339.
[49] 田雪文. 基于iTRAQ的低氧运动肥胖大鼠肝脏蛋白质组学及Wnt/β-catenin信号通路的作用机制研究[D]. 上海:上海体育学院, 2018.
[50] ARNSDORF EJ, TUMMALA P, CASTILLO AB, et al. The epigenetic mechanism of mechanically induced osteogenic differentiation. Biomech.2010;43(15):2881–2886.
[51] VLAIKOU AM, KOUROUPIS D, SGOUROU A, et al. Mechanical stress affects methylation pattern of GNAS isoforms and osteogenic differentiation of hAT-MSCs.Biochim Biophys Acta Mol Cell Res. 2017; 1864(8):1371-1381.
[52] HUM JM, DAY RN, BIDWELL JP, et al. Mechanical Loading in Osteocytes Induces Formation of a Src/Pyk2/MBD2 Complex That Suppresses Anabolic Gene Expression. Plos One.2014; 9(5):e97942. |