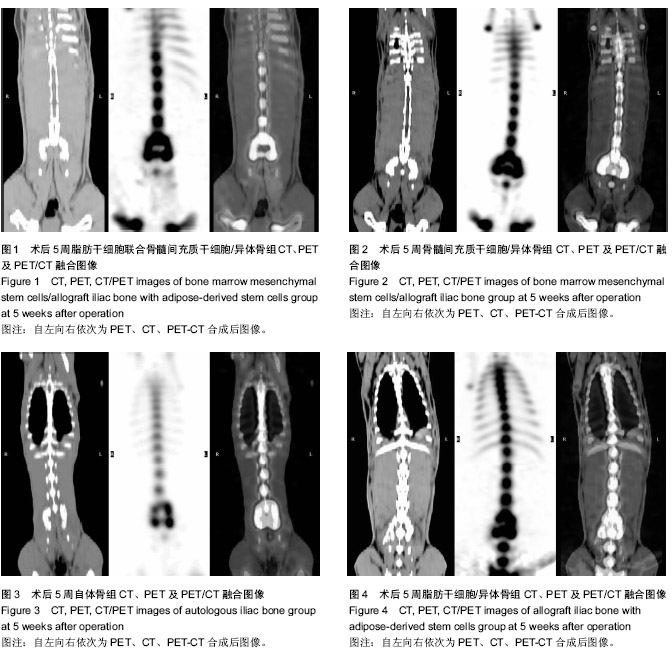
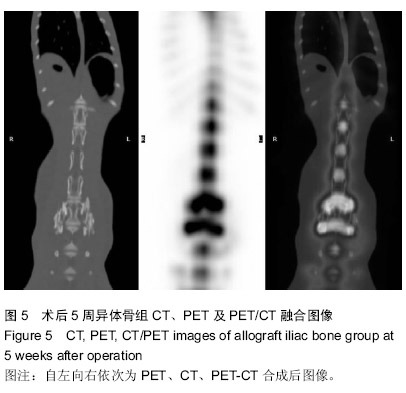
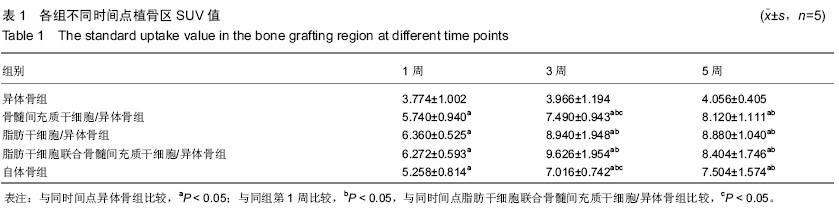
| [1] Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973-3982.
[2] 翟永清,干耀恺,秦安,等. 富集骨髓基质干细胞复合多孔β-磷酸三钙促进骨缺损修复的实验研究[J].中华创伤骨科杂志,2009,11(11):1058-1061.
[3] Zhou C, Cai X, Grottkau BE, et al. BMP4 promotes vascularization of human adipose stromal cells and endothelial cells in vitro and in vivo. Cell Prolif. 2013;46(6):695-704.
[4] Kang BJ, Lee SH, Kweon OK, et al. Differentiation of canine adipose tissue-derived mesenchymal stem cells towards endothelial progenitor cells. Am J Vet Res. 2014;75(7): 685-691.
[5] Eluvathingal Muttikkal TJ, Shami VM, Jones DR, et al. FDG Positron Emission Tomography and Computed Tomography Demonstration of Carcinoma Arising in an Epiphrenic Diverticulum. J Radiol Case Rep. 2014;8(11):42-46.
[6] Luo Z, Liu M, Sun L, et al. Icariin recovers the osteogenic differentiation and bone formation of bone marrow stromal cells from a rat model of estrogen deficiency-induced osteoporosis. Mol Med Rep. 2015;12(1):382-388.
[7] Liang W, Zhuo X, Tang Z, et al. Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol Cell Biochem. 2015;402(1-2):101-110.
[8] Yan SG, Zhang J, Tu Q, et al. Transcription factor and bone marrow stromal cells in osseointegration of dental implants. Eur Cell Mater. 2013;26:263-270.
[9] Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521-2529.
[10] Brodke D, Pedrozo HA, Kapur TA, et al. Bone grafts prepared with selective cell retention technology heal canine segmental defects as effectively as autograft. J Orthop Res. 2006;24(5): 857-866.
[11] de Girolamo L, Niada S, Arrigoni E, et al. Repair of osteochondral defects in the minipig model by OPF hydrogel loaded with adipose-derived mesenchymal stem cells. Regen Med. 2015;10(2):135-151.
[12] Park IS, Mondal A, Chung PS, et al. Vascular regeneration effect of adipose-derived stem cells with light-emitting diode phototherapy in ischemic tissue. Lasers Med Sci. 2015;30(2): 533-541.
[13] Haubner F, Gassner HG. Potential of adipose-derived stem cells concerning the treatment of wound healing complications after radiotherapy. HNO. 2015;63(2):111-117.
[14] Sheng L, Yang M, Li H, et al. Transplantation of adipose stromal cells promotes neovascularization of random skin flaps. Tohoku J Exp Med. 2011;224(3):229-234.
[15] Zhang W, Wray LS, Rnjak-Kovacina J, et al. Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials. 2015; 56:68-77.
[16] Cui N, Qian J, Liu T, et al. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr Polym. 2015;126:192-198.
[17] Thirumala S, Wu X, Gimble JM, et al. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods. 2010;16(4):783-792.
[18] Caroli A, Antiga L, Conti S, et al. Intermediate volume on computed tomography imaging defines a fibrotic compartment that predicts glomerular filtration rate decline in autosomal dominant polycystic kidney disease patients. Am J Pathol. 2011;179(2):619-627.
[19] Brandoff JF, Silber JS, Vaccaro AR. Contemporary alternatives to synthetic bone grafts for spine surgery. Am J Orthop (Belle Mead NJ). 2008;37(8):410-414.
[20] Kurobe H, Maxfield MW, Breuer CK, et al. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med. 2012;1(7): 566-571. |