Design
A comparative observation.
Materials
Electronic mixers, incubator, ultra-quiet bench, BHI Agar, Sabouraud Medium, glucose peroxidase kit, nHA with pH 8, 9, 10, AH-Plus root canal sealer, Vitapex root canal sealer, zinc oxide eugenol, ornidazole and glycerin. Microbial strains: Enterococcus Faecalis (ATCC 29212), Candida Albicans (ATCC 10231), Staphylococcus Aureus (ATCC 25923).
Methods
Antimicrobial test
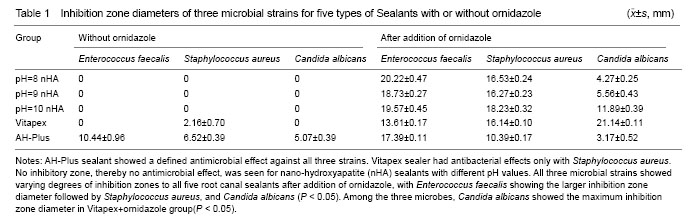
Experiment groups: (1) nHA with pH 8, 9, 10, Vitapex and AH-Plus, totally five groups. (2) Ornidazole was added to the above mentioned five groups at a mass ratio of 7:3 to make another five groups.
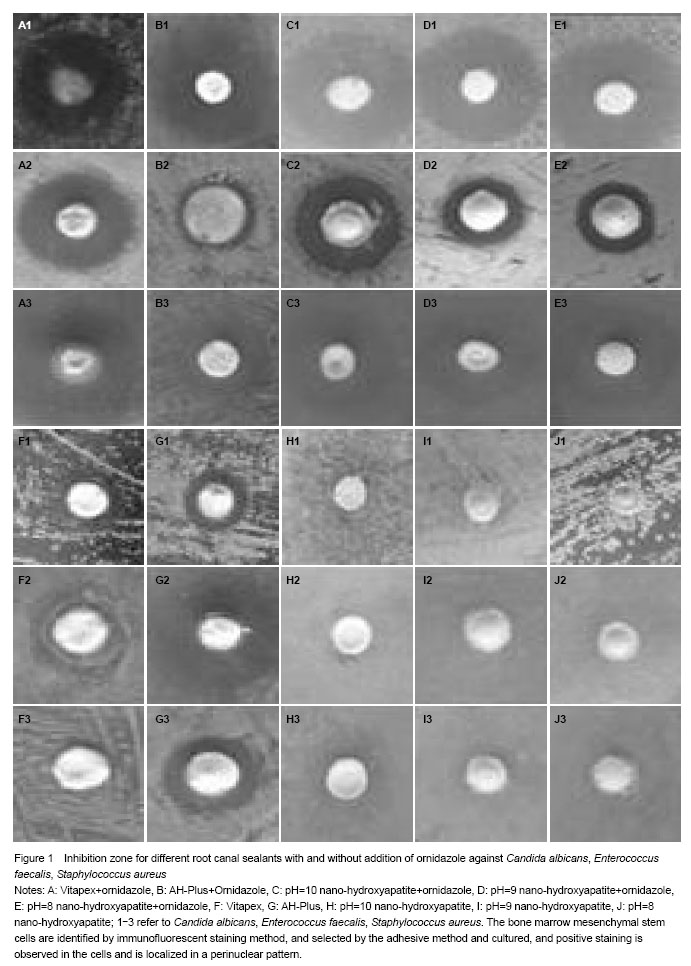
Strain recovery and activation: After individual microbial strain recovery and activation, using Agar diffusion method, we prepared different strains corresponding to the plate medium. Three holes of 0.7 mm in diameter were prepared on each plate and bacterial mediums were uniformly coated on the flat surface of the agar plate.
100 mg of root canal sealants were added to the prepared holes and the plates were inverted and incubated at 37 ℃ for 24 hours to prepare 1×105 CFU/mL bacterial liquid for observations and recording.
Measuring zone diameter and result determination[4-5]: After the plates had been overnight incubated, there should be a noticeable “clearing zone” around each of the materials used. The diameter of each inhibition zone was measured and recorded in millimeters (mm). The criteria for recording the microbial inhibition zone test results are as follows: ≥ 0.5 mm as inhibitory effect and < 0.5 mm as no inhibition which is recorded as 0.
Microleakage test
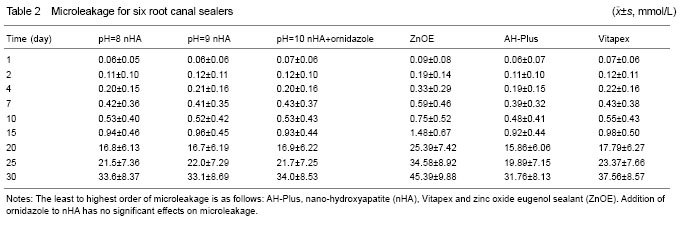
Grouping: This in vitro experiment consisted of 60 extracted single rooted, single-canalled teeth where cleaning and shaping of the canals were performed to file size #40. Five experimental groups were: nHA pH=8, nHA pH=9, nHA pH=10+ornidazole (mass ratio 7:3), AH-Plus and Vitapex sealants and one control group: zinc oxide eugenol sealant.
Root canal filling: Lateral condensation technique was used with gutta percha and the individual group sealant to obturate the canals. The length of the gutta percha points from the canal orifice to the root apex was 10 mm and the excess was removed using #4 Gates Glidden drill. Later, vertical pressure was performed for compression of gutta percha in the canals. Thereafter, three layers of nail polish were applied to the tooth surface, except for the apical
2 mm and the canal orifice, which remained exposed, in such a way that the solution could pass through the canal. Teeth were stored in a 37 ℃, 100% humidity incubator for a week to allow for setting of the sealers. The coronal part of the teeth was fixed and sealed on to the larger opening of an Eppnedorf tube, where two-thirds of the tooth is seen outside the tube opening. The upper part of tube was connected to a transparent infusion tube, through which 0.2% NaN3 and 1 mL/mol glucose solution was passed. The solution should be passed from the length of the infusion tube to the canal orifice where gutta percha was seen, which was around 15 cm. The solution level in the infusion tube was maintained constantly throughout the experiment. The end of the Eppnedorf tube with the tooth was fixed and sealed on to small bottles. A #12 needle was inserted on the bottle cap for atmospheric contact. The complete setup was placed at 37 ℃ saturated humid atmospheres.
10 μL of the samples was collected from the glass bottles at the intervals of 2, 4, 7, 10, 15, 20, 25 and 30 days respectively. 10 μL of distilled water was added to all the glass bottles after completing each day sample collections. With glucose oxidase kit, the automatic biochemical analyzing instrument was used to record the absorbance value at the wavelength of 490 nm and the corresponding glucose concentration was then calculated.