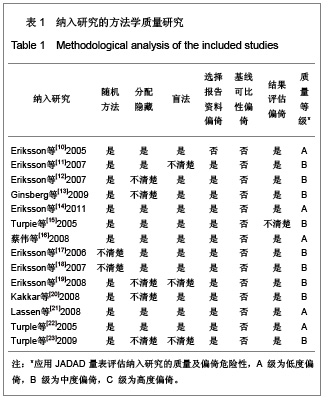
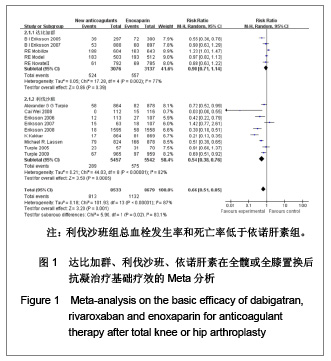
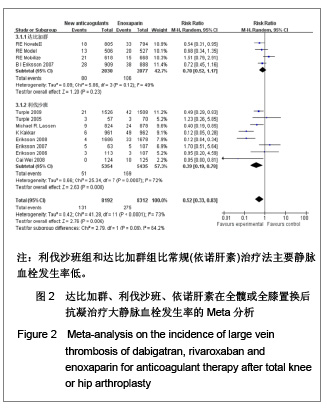
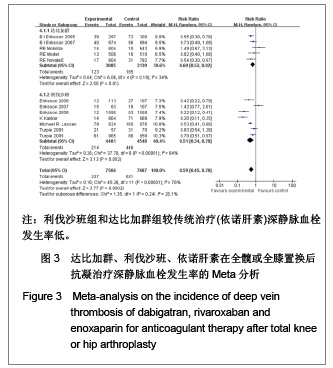
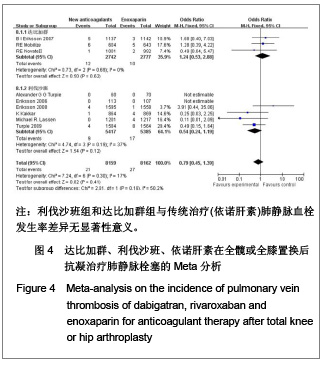
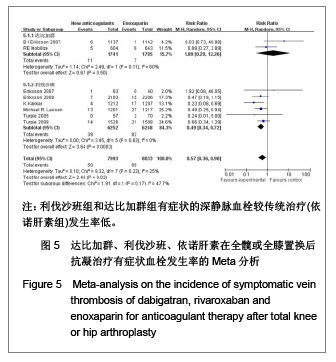
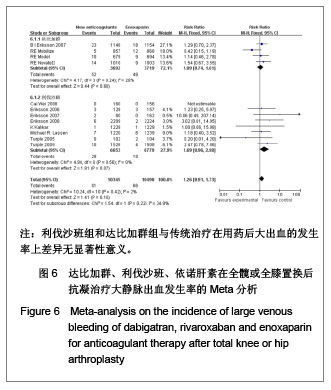
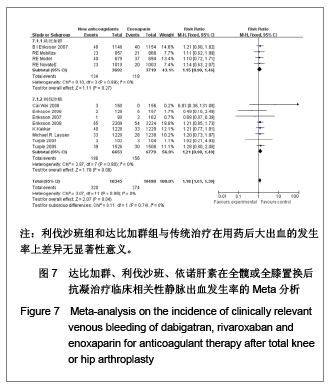
| [1] Sobieraj DM, Coleman CI, Tongbram V, et al. Venous Thromboembolism Prophylaxis in Orthopedic Surgery [Internet]. Cells Tissues Organs. 2003;174(3):101-109.[2] Llau JV, Gil-Garay E, Castellet E, et al. [Thromboprophylaxis with enoxaparin for total knee replacement: An observational, retrospective and multicentre study comparing starting the treatment before and after the operation]. Rev Esp Anestesiol Reanim. 2012;59(6): 306-314.[3] Carrier M, Cushman M. Assessing the utility of venous thrombosis prophylaxis in orthopedic surgery patients. Ann Intern Med. 2012;156(10): 748-749.[4] Bosque J Jr, Coleman SI, Di Cesare P.Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35(3): 228-233; quiz 234-235.[5] Enokiya T, Muraki Y, Iwamoto T, et al. Risk factor for a residual deep vein thrombosis after fondaparinux administration in patient with postoperative replacement arthroplasty. Yakugaku Zasshi. 2012;132(5): 683-687.[6] Aaron RK, Boyan BD, Ciombor DM, et al. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;(419): 30-37.[7] Ufer M. Comparative efficacy and safety of the novel oral anticoagulants dabigatran, rivaroxaban and apixaban in preclinical and clinical development. Thromb Haemost. 2010; 103(3): 572-585.[8] Anderson FA Jr, Zayaruzny M, Heit JA, et al. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82(9): 777-782.[9] Cao YB, Zhang JD, Shen H, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2010;66(11): 1099-1108.[10] Eriksson BI, Dahl OE, Büller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3(1): 103-111.[11] Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5(11): 2178-2185.[12] Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370(9591): 949-956.[13] Ginsberg JS, Davidson BL, RE-MOBILIZE Writing Committee, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24(1): 1-9.[14] Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105(4): 721-729.[15] Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost. 2005; 3(11): 2479-2486.[16] 柴伟,王岩.利伐沙班预防成人全髋关节置换术后静脉血栓栓塞的有效性及安全性研究[D].中国人民解放军军医进修学院, 2008.[17] Eriksson BI, Borris L, Dahl OE, et al. Oral, direct Factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2006;4(1): 121-128.[18] Eriksson BI, Borris LC, Dahl OE, et al. Dose-escalation study of rivaroxaban (BAY 59-7939)--an oral, direct Factor Xa inhibitor--for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res. 2007; 120(5): 685-693.[19] Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26): 2765-2775.[20] Kakkar AK, Brenner B, Dahl OE, et al., Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372 (9632): 31-39.[21] Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358(26): 2776-2786.[22] Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost. 2005;3(11): 2479-2486.[23] Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009; 373(9676): 1673-1680.[24] Rahman S. Deep vein thrombosis prophylaxis: friend or foe. Am J Ther. 2009;16(4): 300-303.[25] Anderson FA Jr, Zayaruzny M, Heit JA, et al. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82(9): 777-782.[26] Galson SK. Prevent deep vein thrombosis and pulmonary embolism with a healthful diet. J Am Diet Assoc. 2009;109(4): 592.[27] Goldhaber SZ, Fanikos J. Cardiology patient pages. Prevention of deep vein thrombosis and pulmonary embolism. Circulation. 2004;110(16): e445-447.[28] Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7): 874-849.[29] Turun S, Banghua L, Yuan Y, et al. A systematic review of rivaroxaban versus enoxaparin in the prevention of venous thromboembolism after hip or knee replacement. Thromb Res. 2011;127(6): 525-534.[30] Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest.2004;126(3 Suppl): 204S-233S. |