| [1] 温稳,张保平,白仲添,等.杨氏模量及细胞骨架重塑对肝癌细胞侵袭的影响[J].中华消化杂志,2015,35(6):371-376.[2] 温斯健,倪娜娜,周晓伟,等.过表达RhoD对人黑素瘤A375细胞骨架和迁移侵袭的影响[J].中华皮肤科杂志, 2016,49(12):871-875.[3] Cross SE,Jin YS, Rao J,et al.Nanomechanical analysis of cells from cancer patients.Nat Nanotechnol. 2007;2(12):780-783.[4] 李玲珺,李霞.基于微管靶点的抗肿瘤药物研究进展[J].中国药师, 2017, 20(1):139-143.[5] Ulrich TA,de Juan Pardo EM,Kumar S.The mechanical rigidity of the extracellular matrix regulates the structure,motility,and proliferation of glioma cells.Cancer Res. 2009;69(10):4167-4174.[6] Peckham M.How myosin organization of the actin cytoskeleton contributes to the cancer phenotype. Biochem Soc Trans. 2016;44(4): 1026-1034.[7] Lopez JI,Kang I,You WK,et al.In situ force mapping of mammary gland transformation.Integr Biol (Camb). 2011;3(9):910-921.[8] Schrader J,Gordon-Walker TT,Aucott RL,et al.Matrix stiffness modulates proliferation,chemotherapeutic response,and dormancy in hepatocellular carcinoma cells.Hepatology. 2011;53(4):1192-1205.[9] Park J,Kim DH,Kim HN,et al.Directed migration of cancer cells guided by the graded texture of the underlying matrix.Nat Mater. 2016;15(7): 792-801 [10] Tilghman RW,Cowan CR,Mih JD,et al.Matrix rigidity regulates cancer cell growth and cellular phenotype.PLoS One. 2010;5(9):e12905.[11] Paszek MJ,Zahir N,Johnson KR,et al.Tensional homeostasis and the malignant phenotype.Cancer Cell.2005;8(3):241-254.[12] Wang N,Ingber DE.Control of cytoskeletal mechanics by extracellular-matrix, cell-shape,and mechanical tension.Biophys J. 1994;66(6):2181-2189.[13] Tang X,Kuhlenschmidt TB,Zhou J,et al.Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J.2010;99(8):2460-2469.[14] Zilman AG,Safran SA.Role of cross-links in bundle formation,phase separation and gelation of long filaments.Europhys Lett. 2003;63(1): 139-145.[15] Besser A,Schwarz US.Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction.New J Physics.2007;9(11):427-451.[16] 石小川,沈超,聂巍,等.非肌肉Ⅱ型肌球蛋白在细胞骨架网络中动力学行为的图像分析[J].生物化学与生物物理进展, 2016,43(3):244-255.[17] Discher DE,Janmey P,Wang YL.Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(18):1139-1143.[18] Yeung T,Georges PC,Flanagan LA,et al.Effects of substrate stiffness on cell morphology,cytoskeletal structure,and adhesion.Cell Motil Cytoskeleton.2005;60(1):24-34.[19] Shutova M,Yang C,Vasiliev JM,et al.Functions of nonmuscle myosin Ⅱ in assembly of the cellular contractile system.PLoS One. 2012; 7(7):e40814.[20] Ridley AJ.Rho GTPase signalling in cell migration.Curr Opin Cell Biol. 2015;36:103-112.[21] Brown TD,Pedersen DR,Baker KJ,et al.Mechanical consequences of core drilling and bone grafting on osteonecrosis of the femoral head. Bone Joint Surg Am. 1993;75(9):1358-1367.[22] Engler AJ,Sen S,Sweeney HL,et al.Matrix Elasticity Directs Stem Cell Lineage Specification.Cell.2006;44(6):677-689.[23] Ingber DE.Tensegrity II.How structural networks influence cellular information processing networks.J Cell Sci. 2003;116(Pt 8):1397-408. [24] Wei Q,Adelstein RS.Conditional expression of a truncated fragment of nonmuscle myosin Ⅱ-A alters cell shape but not cytokinesis in HeLa cells.Mol Biol Cell. 2000;11(10):3617-3627.[25] Beningo KA,Lo CM,Wang YL.Flexible polyacrylamide substrata for the analysis of mechanical interactions at cellsubstratum adhesions. Methods Cell Biol. 2002;69:325-339.[26] Zhang Q,Wang X,Wei X.Characterization of viscoelastic properties of normal and osteoarthritic chondrocytes in experimental rabbit model. Osteoarthritis Cartilage. 2008;16(7):837-840.[27] 徐晋斌,樊学军,张火圣,等.微管吸吮和半无限体模型在鼠成骨细胞粘弹性研究中的应用[J].生物物理学报,1998,14(2):360-367.[28] Senapati SK,Pal S.UHMWPE-alumina ceramic composite: A proposed metal substitute for artificially replaced hip Joint.J Inst Eng(India). 2005; 85:157-162.[29] Zhu Y,Yan ZQ,Shen BR,et al.Effect of Akt/PKB activation on mechanical strain-induced vascular smooth muscle cell migration.J Med Biomech.2006;21(4):259-261.[30] Xu BY,Song GB.Advances in selective differentiation of bone marrow mesenchymal stem cells induced by mechanical stimulation.J Med Biomech.2007;22(1):104-108.[31] Zhou T,Wang CH,Yan H,et al.Inhibition of the Rac1-WAVE2-Arp2/3 signaling pathway promotes radiosensitivity via downregulation of cofilin-1 in U251 human glioma cells.Mol Med Rep. 2016;13(5): 4414-4420.[32] Carlson RO.New tubulin targeting agents currently in clinical development.Expert Opin Investig Drugs.2008;17(5):707-722[33] Ulrich T A,de Juan Pardo E M, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility,and proliferation of glioma cells.Cancer Res. 2009;69(10):4167-4174.[34] Tilghman RW,Blais EM,Cowan CR,et al.Matrix rigidity regulates cancer cell growth by modulating cellular metabolismand protein synthesis. PLoS One. 2012;7(5):e37231.[35] Lu P,Weaver VM,Werb Z.The extracellular matrix: a dynamic niche in cancer progression.J Cell Biol. 2012;196(4):395-406.[36] Paszek MJ,Zahir N,Johnson KR,et al.Tensional homeostasis and the malignant phenotype.Cancer Cell.2005;8(3):241.[37] Levental KR,Yu H,Kass L,et al.Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin signaling.Cell. 2009;139(5): 891-906.[38] Paszek MJ,Zahir N,Johnson KR,et al.Supplemental data Tensional homeostasis and the malignant phenotype.Cancer Cell. 2005;10(6): 121-124.[39] Tilghman RW,Cowan CR,Mih JD,et al.Matrix Rigidity Regulates Cancer Cell Growth and Cellular Phenotype.PloS One. 2010;5(9): e12905.[40] Tang X,Kuhlenschmidt TB,Zhou J,et al.Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J.2010;99(8):2460. [41] Cheffings TH,Burroughs NJ,Balasubramanian MK. Actomyosin Ring Formation and Tension Generation in Eukaryotic Cytokinesis.Curr Biol. 2016;26(15):719-737.[42] Matsuoka T,Yashiro M.Rho/ROCK signaling in motility and metastasis of gastric cancer.World J Gastroenterol. 2014;20(38):13756-13766.[43] Martin SK,Kamelgarn M,Kyprianou N.Cytoskeleton targeting value in prostate cancer treatment. Am J Clin Exp Urol. 2014;2(1):15-26.[44] Wang W,Eddy R,Condeelis J.The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7(6):429-440.[45] Zhang Q,Liao X,Tang W,et al.Adhesion of nasopharyngeal carcinoma cells to hyaluronic acid and morphological observation of microfilament bylLaser confo cal scanning microscopy.Chin J Histochem Cytochem. 1997;6(1):82-86.[46] Shih JY,Lee YC,Yang SC,et al.Collapsin response mediator protein-1:a novel invasion-suppressor gene.Clin Exp Metastasis.2003;20(1):69-76 |
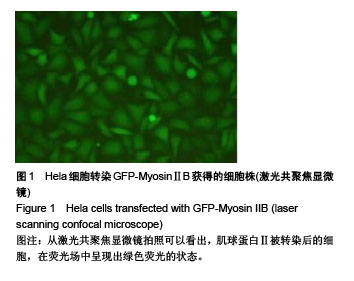
.jpg)
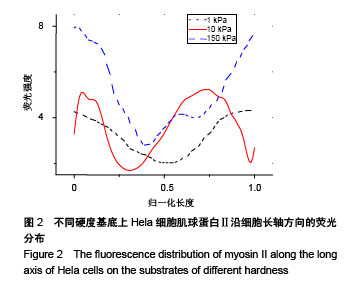
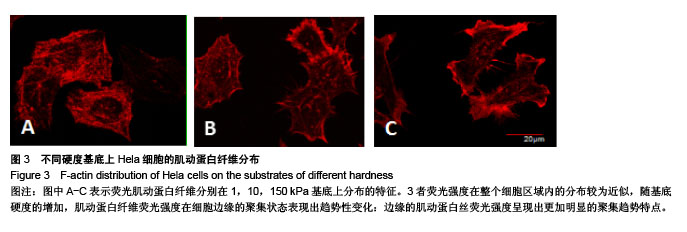
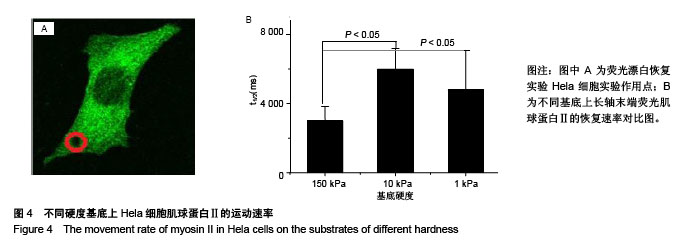
.jpg)
.jpg)