中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (15): 2452-2460.doi: 10.12307/2024.255
• 生物材料综述 biomaterial review • 上一篇
骨微环境对组织工程骨再生过程的影响
钟思扬1,廖 晴1,周星宇1,李先楹1,卫晶晶2,杨 琳2
- 1遵义医科大学珠海校区,广东省珠海市 519041;2遵义医科大学珠海校区人体解剖学教研室,广东省珠海市 519041
-
收稿日期:2023-02-20接受日期:2023-03-25出版日期:2024-05-28发布日期:2023-09-23 -
通讯作者:杨琳,博士,副教授,遵义医科大学珠海校区人体解剖学教研室,广东省珠海市 519041 -
作者简介:钟思扬,女,2002年生,广东省江门市人,汉族,主要从事骨组织工程和神经功能重建研究。 廖晴,女,2002年生,广西壮族自治区玉林市人,汉族,主要从事骨组织工程方面的研究。 -
基金资助:国家自然科学基金项目(82260456,81960419),项目负责人:杨琳;遵义医科大学2022年国家级大学生创新创业训练计划项目(202210661029),项目负责人:周星宇;遵义医科大学2022年省级大学生创新创业训练计划(S202210661043X),项目负责人:李先楹;遵义医科大学2022年校级大学生创新创业训练计划(ZXCH2022003);遵义医科大学研究生教育创新计划项目(ZYK89),项目负责人:卫晶晶
Influence of bone microenvironment on regeneration process of tissue-engineered bone
Zhong Siyang1, Liao Qing1, Zhou Xingyu1, Li Xianying1, Wei Jingjing2, Yang Lin2
- 1Zhuhai Campus, Zunyi Medical University, Zhuhai 519041, Guangdong Province, China; 2Department of Human Anatomy, Zhuhai Campus, Zunyi Medical University, Zhuhai 519041, Guangdong Province, China
-
Received:2023-02-20Accepted:2023-03-25Online:2024-05-28Published:2023-09-23 -
Contact:Yang Lin, PhD, Associate professor, Department of Human Anatomy, Zhuhai Campus, Zunyi Medical University, Zhuhai 519041, Guangdong Province, China -
About author:Zhong Siyang, Zhuhai Campus, Zunyi Medical University, Zhuhai 519041, Guangdong Province, China Liao Qing, Zhuhai Campus, Zunyi Medical University, Zhuhai 519041, Guangdong Province, China -
Supported by:National Natural Science Foundation of China, No. 82260456, 81960419 (to YL); National-Level Training Program for Students’ Innovation and Entrepreneurship of Zunyi Medical University in 2022, No. 202210661029 (to ZXY); Provincial-Level Training Program for Students’ Innovation and Entrepreneurship of Zunyi Medical University in 2022, No. S202210661043X (to LXY); University-Level Training Program for Students’ Innovation and Entrepreneurship of Zunyi Medical University in 2022, No. ZXCH2022003; Postgraduate Education Innovation Program Project of Zunyi Medical University, No. ZYK89 (to WJJ)
摘要:
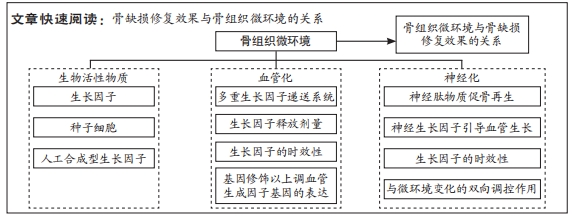
文题释义:
骨组织工程:通过结合种子细胞、组织工程材料及物理化学因子等要素来调控骨组织微环境的变化以诱导新的功能性骨再生,提升或替代受损骨组织器官的生物学功能。骨组织微环境:是指能够近距离作用和调控骨组织细胞兴奋或抑制活动的微量理化因素或生物因素及骨组织细胞在分化过程中形成的、对相关细胞因子敏感的特殊结构和受体所组成的综合性功能环境。
背景:骨组织缺损是目前骨科最为常见的疾病之一,并且该疾病现行的治疗手段均存在一定的不足。组织工程的发展为骨缺损修复带来了新的希望,通过调控缺损部位生物活性物质的释放和血管化、神经化的进程可以有效改善骨组织微环境并促进骨整合,是大尺寸骨缺损修复最具发展潜力的研究思路。
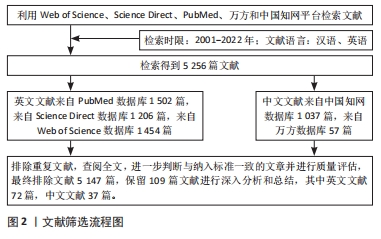
目的:从生物活性物质、血管再生和神经化对骨微环境变化3个方面的影响,探讨近年来调控骨微环境变化在骨缺损修复中的研究进展,为治疗大尺寸骨缺损提供新的思路和策略。方法:在中国知网和万方数据库分别以“骨组织工程,血管生成,神经化,细胞因子,骨形态发生蛋白,血管内皮生长因子,神经肽,骨微环境”为检索词;在Web of Science,Science Direct,PubMed数据库分别以“bone tissue engineering,angiogenesis,neurotization,cytokines,bone morphogenetic protein,vascular endothelial growth factor,neuropeptide,bone microenvironment”为检索词,检索 2001-01-01/2022-12-31收录的有关骨微环境变化的影响因素及在骨组织工程中的应用研究,最终纳入109篇文献进行综述分析。
结果与结论:①骨微环境是诱导骨组织干细胞生长分化的重要保障,其主要包括骨组织种子的细胞外基质及细胞间的相互作用所需要的生物化学因子、局部血液循环网络和周围的神经组织。②骨缺损修复是一个分为多个阶段的连续过程,这些阶段相互重叠,由多种细胞因子介导,同一种细胞因子在一个或多个愈合阶段可以产生相互协同或拮抗的作用。③新生血管再生是启动骨修复的关键,新生血管不仅为骨修复提供了必需的营养物质、成骨细胞和生长因子,同时更是修复细胞进入损伤区的通道。④除了调控血管诱导因子的释放种类、剂量及时效性等因素以实现血运重建外,多因子差异性释放递送系统的研究和基因转移技术的应用将是未来解决大面积骨缺损的研究方向。⑤神经肽类物质能与相关受体结合并作用于特定的信号通路,通过多种途径引导血管的生长及影响骨愈合、骨再生及成骨与破骨之间的平衡。⑥在建立神经化的组织工程骨时,骨组织微环境变化与神经调控的作用是双向的。骨基质中的细胞因子可以通过血神经屏障参与神经元的信号传导通路。而由神经胶质细胞分泌的神经肽类物质能作用于骨微环境,影响骨愈合、骨再生及成骨与破骨之间的平衡。⑦关于生物活性物质和血管化、神经化的进程对骨微环境的调控还存在诸多尚待解决的问题,如细胞因子在人体内弥散和降解速度过快而易丧失活性、血管生成相关生长因子的时效性及空间分布、通过机体回馈调节机制建立神经化等诸多问题,还需后续研究不断完善。
https://orcid.org/0000-0003-3884-6522 (钟思扬);https://orcid.org/0000-0003-4348-0908 (廖晴);https://orcid.org/0000-0003-1372-8677 (杨琳)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号:
引用本文
钟思扬, 廖 晴, 周星宇, 李先楹, 卫晶晶, 杨 琳. 骨微环境对组织工程骨再生过程的影响[J]. 中国组织工程研究, 2024, 28(15): 2452-2460.
Zhong Siyang, Liao Qing, Zhou Xingyu, Li Xianying, Wei Jingjing, Yang Lin. Influence of bone microenvironment on regeneration process of tissue-engineered bone[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(15): 2452-2460.
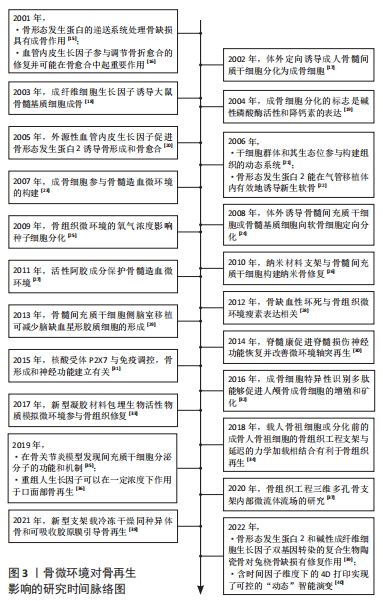
2.1 生物活性物质与骨缺损修复 骨缺损修复是一个分为多个阶段的连续过程,这些阶段相互重叠,由多种细胞因子介导,同一种细胞因子在一个或多个愈合阶段可以产生相互协同或拮抗的作用。在骨愈合过程中,细胞因子形成一个复杂而有序的网络。细胞因子既是骨组织微环境的组成部分,也是骨组织修复和保持骨组织微环境稳态的调节剂,对于骨再生起着核心的调控作用。骨组织重建修复在各个阶段均需要借助精细的调控来完成成骨过程,在此过程当中需要多种化学信号分子参与信息调控,这些化学信号分子包括全身激素如甲状旁腺激素、糖皮质激素和性激素等对骨形成均有影响。近年来,骨组织局部生长调节因子对骨组织的修复机制成为研究热点[41-42]。
2.1.1 生长因子 是一类可以诱导多能细胞增殖和分化的细胞因子,是信息传导过程中的重要介质,在调整相关蛋白质表达方面发挥重要作用。目前认为与骨再生有关的生长因子主要包括血小板衍生生长因子、血管内皮生长因子、成纤维细胞生长因子、骨形态发生蛋白、转化生长因子β、胰岛素样生长因子和血小板衍生生长因子BB等。
生长因子会对信号分子的协同参与和信息表达产生一定的影响,它们在组织再生以及调整相关蛋白表达等方面发挥重要的调节作用[43]。例如,转化生长因子β参与控制骨组织的稳态和重塑,被认为是最主要的骨重塑介质之一[44]。目前受到广泛关注的细胞因子骨形态发生蛋白是转化生长因子β超家族的成员之一,具有诱导成骨细胞分化的能力[45]。骨形态发生蛋白2,4,5,6,7能与Ⅰ型和Ⅱ型丝氨酸-苏氨酸激酶受体结合,激活细胞内信号传导蛋白(Smad)和丝裂原活化蛋白激酶(mitogen-activated protein kinases,MAPK)途径,具有显著的诱导成骨作用[46]。骨形态发生蛋白2能够诱导骨髓间充质干细胞向成骨细胞分化,而骨形态发生蛋白7可以直接促进血管生成[47]。此外,血管内皮生长因子也是一个已经被广泛研究的关键生长因子,它可以介导血管的形成,并在血管生成和骨重塑中发挥调节作用[48-49]。此外,其他生长因子在调节骨组织平衡方面也发挥着至关重要的作用[50]。
2.1.2 种子细胞 是组织工程骨微环境的重要组成部分。种子细胞是一种可分化为多种骨组织细胞的干细胞,其可通过分泌多种细胞因子、骨基质参与骨组织细胞的免疫应答、刺激造血及促进骨组织细胞增殖分化等多种生理活动。因此,种子细胞常被植入组织工程材料中以提高该材料的生物活性及成骨效果。目前应用较为广泛的骨组织工程的种子细胞有成骨细胞、软骨细胞、骨髓间充质干细胞、脂肪干细胞、胚胎干细胞、诱导多能干细胞和经血源子宫内膜干细胞等[51]。
种子细胞通过分泌细胞生长因子从而参与一系列成骨信号的信息调控过程。骨膜来源干细胞是骨组织工程领域中使用频率较高的种子细胞,其能同时释放可以控制细胞增殖及分裂的成纤维细胞生长因子2和诱导骨外膜细胞向软骨分化的骨形态发生蛋白2 [52],该种子细胞具有成骨、成软骨及成脂分化的潜能,是构建具备高度生物活性的组织工程骨重要的活性物质来源。然而,骨缺损发生后人体会释放大量趋化因子和致炎因子,这些因子通过诱导机体内的成骨前体细胞向损伤部位迁移从而进行组织修复。有研究发现间充质干细胞能通过分泌CC类趋化因子(CC motif chemokine,CC)作用于基质细胞衍生因子1(stromal cell-derived factor-1,SDF-1)/ 受体-趋化因子受体4(C-X-C motif chemokine receptor 4,CXCR4)和血清单核细胞趋化蛋白1(monocyte chemoattractant protein-1,MCP-1)/ CC趋化因子受体2(recombinant chemokine C-C-motif Receptor 2,CCR2)两条信号通路轴,在宿主细胞的募集过程中发挥作用,通过募集宿主成骨相关细胞的间接方式促进骨缺损修复[53]。此外,基质细胞衍生因子、骨形态发生蛋白、胰岛素样生长因子和转化生长因子β等多种相关细胞因子能通过Smad、磷脂酰肌醇3-激酶(PI3K,phosphatidylinositol 3 kinase)-akt (protein kinaseB,AKT)依赖性和c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)/细胞外调节蛋白激酶(extracellular signal regulated kinases,ERK)等信号通路相互调节形成一个系统化的复杂信息传递网络,共同调控骨组织细胞的增殖分化。
种子细胞还可分泌骨基质,并与骨基质一同构建缺损部位的微环境。种子细胞的微环境主要包括骨组织种子的细胞外基质、细胞间相互作用所需的生物化学因子、局部血液循环网络以及周围的神经组织。目前,骨损伤修复领域使用频率较高的种子细胞为成骨细胞,其分泌的骨基质能作为细胞和细胞、细胞和细胞质之间信号传导的桥梁[54]。骨基质中富含多种蛋白质,还包括如生长因子、蛋白多糖及微基质蛋白等特殊蛋白,它们共同构成骨组织细胞微环境的网络结构,这些结构物质能共同调节骨基质内相应的温度及酸碱度等理化性质从而为细胞生长或是相关蛋白质的信息表达提供适应性的微环境[55]。细胞外基质含有Ⅰ,Ⅲ,Ⅳ,Ⅴ,Ⅶ等各型胶原,这些结构基础对于细胞中基因表达的方式如细胞的支撑、黏附及移行等行为都有重要的调控作用。
此外,种子细胞的增殖分化也受到细胞因子的调控。种子细胞具有多向分化能力,可被诱导分化为纤维软骨细胞等多种骨组织功能细胞。骨髓间充质干细胞具有良好的软骨分化潜能,因而常被选为组织工程中的种子细胞[56]。杨均等[57]发现转化生长因子β中的转化生长因子β1,β3两种亚型的细胞因子具有诱导骨髓间充质干细胞向透明软骨细胞分化和向纤维软骨细胞分化的能力。
因此,不难发现在上述的种子细胞参与构建微环境机制中,种子细胞通过分泌细胞因子和细胞基质参与骨组织微环境的构建,其自身的增殖分化也受到多种生长因子的影响,这些微环境成分的相互作用是构建有机成骨调控体系的基础。
2.1.3 人工合成型生长因子物质 骨缺损部位的骨再生和重塑需要依赖微环境中一系列的生物化学信号分子的调节,如细胞生长因子和种子细胞等。信号通路的中断会影响种子细胞的分化从而减弱骨修复重建效果。由人工设计并合成且来源于生长因子的多肽可作为生长因子的替代物,为骨组织细胞创造良好的生物活性微环境[58]。
细胞因子是由某些骨组织细胞或是非骨组织细胞受到刺激而合成并分泌的小分子多肽。已有研究表明,人工合成的短链多肽在多种化学分子参与的信号传导通路上能充分暴露活性位点并与细胞表面受体结合。细胞因子是信号传导通路中的衔接蛋白,含有各种能与其他蛋白多肽结合的结构域,并通过特异性的蛋白质-蛋白质相互作用形成复合体激活下游的信号通路。因而人工合成具有特定功能的多肽蛋白不仅能有效避免其作用于蛋白中多种多肽作用的非受体靶点,还可以通过化学修饰调节多肽结构以提高其在复合过程的稳定性。人工设计的蛋白多肽作用专一、生物活性更强且易于大规模合成[59],因此,人工合成型生长因子物质具有与细胞因子相似的作用特性,能近距离调控骨组织细胞兴奋从而参与构建骨组织微环境。
目前,使用人工合成的生物化学活性物质替代诱导骨组织干细胞生长分化的生长因子的研究已经取得了一定进展。相关研究发现,Wnt信号通路是目前骨缺损修复研究领域的热点,这一通路上的相关调控因子机制在调节骨分化和骨组织代谢的过程中发挥重要的作用[60]。吴穹[61]设计了5段抑制因子(Dickkopf-1,DKK1)的功能多肽并对其进行了体内及体外实验,实验结果证实,部分DKK1多肽可以作为胞外信号因子发挥Wnt蛋白的作用,与细胞膜受体(low density lipoprotein receptor-related proteins 5/6,LRP5/6)相结合,通过抑制小鼠成肌细胞C2C12和小鼠胚胎成骨细胞前体细胞MC3T3-E1细胞中碱性磷酸酶的活性进而影响成骨相关靶基因的转录和表达,进而促进了骨的形成,这项研究证实了人工合成的多肽具有与Wnt信号通路中负向调节因子的相同调节功能。
生物活性物质在骨组织工程领域应用的挑战主要包括其不稳定性、易失活、半衰期短、代谢快以及在局部制剂中迅速失活,这就需要对生物活性物质的持续释放系统(包括剂量、比例、递送时间及空间分布等)进行进一步的科学研究。生物活性物质的控释系统将能够防止其在局部的快速释放,并为缺损部位提供缓慢和稳定的输送。此外,开发具有特定部位释放特性的改进型控释给药系统以提高生物活性物质的生物利用率有较高的研究价值,可以通过利用各种天然或合成聚合物材料的特殊性能或通过开发具有特殊性能的新型纳米聚合物来实现。生物活性物质修复骨缺损的作用研究见表1。

2.2 骨缺损与血管化 骨组织是一个高度血管化的组织。在应用组织工程骨治疗骨缺损时,及时和丰富的血管再生是保证骨缺损修复和组织工程骨存活的基础,血运重建的效果决定了植入组织工程骨后骨缺损部位骨再生和骨端融合的速度和效果[62]。在骨损伤后的修复过程中,新生血管再生是启动骨修复的关键。新生血管不仅为骨修复提供了必须的营养物质、成骨细胞和生长因子,同时更是修复细胞进入损伤区的通道。尤其对于大面积骨缺损,如果缺损部位附近没有完整血管,血管内的循环干细胞就无法通过趋化作用迁移到缺陷中,导致缺损部位的成骨不足及组织工程骨的整合失败[63]。为了解决此问题,CHEN等[64]通过使用内皮细胞和间充质干细胞封装的生物墨水,成功地在三维结构中构建了致密的微血管系统。他们遵循血管生成早于成骨的原则,在构建血管系统后添加成骨细胞,能有效改善骨缺损患者的类骨质沉积及骨体积分数,并形成血管的吻合[65]。GRELLIER等[66]研究表明,缺氧环境下大面积骨缺损部位中心的缺血,导致组织工程骨中的单纯骨髓间充质干细胞不能被诱导分化为成骨细胞,因此早期血管化对骨缺损修复至关重要。
2.2.1 多细胞因子差异性释放呈递系统 在骨的自然骨愈合过程中,血管生成几乎贯穿于愈合的全过程。血管化的建立对提供营养物质和因子以支持骨修复相关细胞的生物过程具有重要意义。而血管生成因子对血管的生成具有调节作用,研究人员必须根据相关因子的不同种类、不同剂量、不同作用时间等状态模式进行因子的递送。血管内皮生长因子具有刺激血管内皮细胞的增殖和迁移的能力,但同时其对周细胞(如血管平滑肌细胞)的生物功能有抑制作用,血管内皮生长因子的持续表达可能导致血管不稳定以及渗漏[62]。尽管血管内皮生长因子具有良好的成骨及成血管功能,但是单独递送血管内皮生长因子并不能实现很好的成骨效果,因此在构建骨组织血管生成的进程上往往需要多重因子的共同作用。SAIK等[67]发现联合使用共价固化的血小板衍生生长因子BB及共价固化的成纤维细胞生长因子2,相较于使用单因子,可以明显提高水凝胶材料中内皮细胞的迁移程度及功能性微血管的生成。PATEL等[68]发现同时递送双细胞因子骨形态发生蛋白与血管内皮生长因子时,5/8的大鼠在12周内骨缺损完全愈合,单独使用骨形态发生蛋白2只有3/8的大鼠存在骨愈合效果。尽管细胞因子骨形态发生蛋白2具有良好的成骨特性,但因其在体内的弥散及降解速度较快,降低了其在缺损部位的局部浓度和治疗效果。然而,单独使用血管内皮生长因子时并没有出现骨缺损的愈合,这表明共同释放双因子对于骨愈合的作用明显强于单因子的递送效果。
此外,研究人员发现,在骨愈合的早期阶段血管内皮生长因子基因的表达较成骨因子骨形态发生蛋白2的表达高峰要早,因此在双细胞因子递送中选择优先递送血管内皮生长因子能获得更好的血管生成以及成骨效果[69-70]。然而,YOUNG等[71]研究发现当设置双因子血管内皮生长因子/ 骨形态发生蛋白2的比率大于1时,同时递送血管内皮生长因子和骨形态发生蛋白2在12周时的骨组织愈合效果不如单独递送骨形态发生蛋白2,这说明在建立血管化时不但要探究不同生长因子之间的相互作用问题,还要注意双重输送或多重输送对骨再生的剂量效应,任何一种因子释放的量不足或是比例选择不当都会干扰体内正常骨的形成[72-75]。由于体内血管生成和血管稳定需要多种生长因子之间的精确协调,以刺激所需的细胞反应。目前研究焦点较少集中于对生长因子剂量效应的确定,但对不同剂量的进一步研究有助于阐明生物活性物质的作用机制。因此,未来进一步的研究应集中于评估对血管和骨形成的剂量依赖性影响。
2.2.2 生物活性物质的时效性 除了血管诱导因子的释放种类和剂量外,血管诱导因子的时效性也至关重要。在骨组织活性支架融合进入机体的初期,促血管生成因子还未发挥诱导种子细胞发生增殖分化形成血管,使得执行运输氧气功能的血红蛋白无法到达缺损部位,从而导致骨组织支架还处于缺氧状态,影响造血干细胞和神经干细胞的增殖分化[76]。AMARILIO等[77]发现在低氧条件下,敲除肢芽间充质中灭活转录因子缺氧诱导因子1α (hypoxia-inducible factor 1α,HIF-1α)后调控小鼠软骨细胞分化的关键调节因子(SRY-related HMG box gene 9,Sox9)表达降低,软骨形成显著减少。这些实验结果说明了干细胞在不同生长阶段内会因为骨组织微环境的含氧量不同而产生不同的表达结果,进而影响骨组织活性支架对骨缺损的修复效果。
2.2.3 基因工程技术的应用 随着近年来组织工程和基因工程的研究进展,将血管内皮生长因子编码基因导入细胞的基因修饰成为重建血循环最具研究前景的治疗模式之一。目前有研究发现应用基因转移技术对种子细胞进行修饰能有效提高目的蛋白的表达。张向荣[78]将外源基因血管内皮生长因子165脂质体以介导方式转入组织工程的种子细胞骨髓间充质干细胞中,移植转染后的骨髓间充质干细胞可使血管内皮生长因子165基因及其蛋白表达上调,有效促进局部血管再生。此外,研究人员通过MTT法对转染后的骨髓间充质干细胞进行测定发现种子细胞骨髓间充质干细胞的增殖和活力不受影响。然而,尽管通过血管内皮生长因子基因转染骨髓间充质干细胞能提高血管内皮生长因子的表达,但其应用于其他种子细胞上时仍存在影响种子细胞的繁殖分化能力及传代能力,导致种子细胞本身发挥的作用降低的可能。
2.2.4 4D生物打印技术的应用 在组织愈合过程中,细胞微环境的动态和广泛重塑通过不断向细胞施加细胞内及细胞外收缩力,进一步触发基因表达和细胞行为的变化,其依赖时间的转换应该是可预测并且是可编程的。理想的组织工程支架应该能够响应这种动态变化,以实现微环境变化与人体的整合。
4D生物打印是近年来的研究热点之一。目前应用最为广泛的的生物打印是在三维空间中生成排列细胞和生物分子的结构[79]。而4D生物打印则是将因子时间作为第四维度添加到生物打印的空间维度中。而生物打印物体因细胞-细胞融合的结构特性或受到特定的外界刺激,可能会改变其功能或形状[80]。例如,打印成平面结构的生物打印材料在接种细胞后经特定的刺激可卷曲或折叠成三维形状。触发形状变化的刺激可以是特定的pH值、电场、磁性、光照、添加化学催化剂或类似刺激的组合[81]。4D生物打印可改变生物打印结构形状的特性可以有效克服常规3D打印的局限性。文章总结了骨缺损部位血管化的机制,见表2。


2.3 神经化与骨再生 骨组织是神经系统重要的靶器官,神经元可整合机体内外环境因素从而对骨的正常代谢以及骨的愈合重建进行调控[82]。近年来大量的研究证明骨的再生与重建不仅受全身激素水平与局部细胞因子的调控,还受到神经系统的调控[83]。机体骨折后,生长因子和细胞因子将骨祖细胞募集到缺损部位。随着骨骼重塑,血管生成允许血管网络侵入损伤部位以恢复正常循环,以替换坏死的骨组织,神经纤维及其分泌的物质(包括信号蛋白、神经营养素及神经肽等)与血管再生密切相关。神经再支配发生在血运重建之前,有助于血管再生。
骨组织内部的大多数交感神经和感觉神经多与血管伴行,广泛分布于骨髓腔、骨膜、骨髓及骨小梁中[84]。有国内外的相关研究发现,神经长入与骨生长发育过程中存在时间上重叠的特点[85-86]。感觉神经和交感神经在正常骨骼代谢和骨缺损修复中发挥重要的调节作用[87-88]。在切除交感神经的动物模型中,出现骨吸收增加、骨沉积和矿化减少的现象,导致骨缺损修复效果较差[89-90]。此外,在关节炎小鼠动物模型中,切除交感神经还可能导致软骨下骨增厚[91-92]。
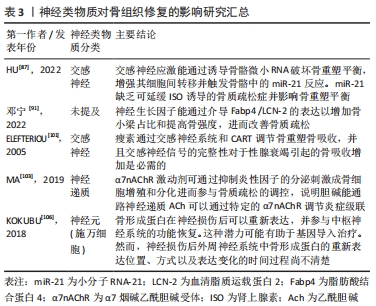
2.3.1 神经肽类物质的作用 骨组织中的神经纤维主要为肽能神经,其主要由分泌降钙素基因相关肽(calcitonin gene-related peptide,CGRP)的感觉神经和分泌血管活性肠肽(vasoactive intestinal peptide,VIP)神经肽Y (neuropeptide tyrosine,NPY)的自主神经构成。神经肽类物质是由神经系统释放并发挥其对骨生物学效应的关键性物质,包括细胞因子、神经营养因子、神经递质以及各种激素。神经肽类物质能与相关受体结合并作用于特定的信号通路,通过多种途径影响骨愈合、骨再生及成骨与破骨之间的平衡。其中降钙素基因相关肽CGRP是一种重要的神经递质,同时也是强效血管活性物质的一种,其可以通过与炎性因子的相互作用以增强血管通透性及促进血管扩张,从而调节血管中的血流变化[93-94]。此外,感觉神经分泌的神经肽对于建立血管化也发挥重要的调节功能。姚旺祥等[95]将感觉神经植入组织工程骨以评估其对大段骨缺损的修复效果时发现组织工程骨中神经肽CGRP、NPY、P物质(substance P,SP)及其受体CGRP1R,NPY1R,MK1水平上调。这些结果说明感觉神经可通过分泌神经肽类物质如SP促进骨髓间充质干细胞的成骨分化和成血管分化,进而促进骨形成。
2.3.2 神经生长因子对血管及骨再生的调控 神经生长因子可以引导血管生长,比较经典的轴突导向因子包括信号素3A(Semaphorin 3A,Sema3)、神经轴突导向因子(Netrins)、磷酸化神经细胞鸟苷酸置换因子(Epherins)等[96-97]。大量文献证实,这些因子结合相应受体后,可以引导血管长入[98]。在胚胎发育期间,神经生长因子/酪氨酸激酶受体A(NGF/TrkA)信号通过诱导感觉神经支配促进血管化[99],而神经生长因子可能引起内皮细胞增殖和炎症。
此外,在建立组织工程骨神经化的进程中,神经生长因子多肽具有指导骨组织细胞生长增殖和分化的功能。姚洋等[100]研究发现体内局部注射神经生长因子能加速小鼠腿骨内钛种植体新生骨胶原的早期成熟,并且促进局部神经元中降钙素基因相关肽(CCRP)的生成和神经元存活。近年来,有研究发现交感神经和感觉神经通过参与多种信号途径对骨组织细胞进行调控,如交感神经信号、乙酰胆碱类信号及激素类等信号。肾上腺素能信号可以通过突触后的肾上腺素能受体(adrenergic receptor,AR)增加成骨细胞核因子κB受体活化因子配体(receptor activator for nuclear factor-κB ligand,Rankl)的表达从而间接刺激破骨细胞的分化[101]。而甲状旁腺素可以作用于细胞因子受体骨保护素 [102],可在破骨细胞发育的后期抑制其形成,能作为激动剂上调成骨细胞中Rankl的表达,从而促进破骨细胞的骨吸收作用。MA等[103]发现交感神经系统能通过增加骨组织中的破骨细胞分化因子Rankl的表达来促进骨吸收。JIAO等[104]在对骨性关节炎动物模型的研究中,发现骨软骨交接区交感神经分支能释放去甲肾上腺素(norepinephrine,NE)信号,并作用于软骨下间充质干细胞上的β2肾上腺素能受体(β2-adrenergic receptors,β2-AR)以及软骨细胞上的α2肾上腺素能受体(α2-adrenergic receptors,α2-AR),进一步通过参与相关的信号传导通路促进骨修复。
2.3.3 骨组织微环境与神经调节的双向调控作用 在神经化组织工程骨的构建过程中,骨组织微环境变化与神经调控的作用是双向的,因此在关注外周神经对骨调控作用的同时还需关注骨组织微环境对外周神经的调控作用,主要体现在骨组织对外周神经功能的调控上。有研究发现,在异位骨化的疾病过程中骨基质会释放骨形态发生蛋白,此过程会改变神经细胞膜的通透性,使得周围神经的血神经屏障开放,导致成骨祖细胞离开神经并迁移到骨化位点,从而发挥对神经的调控作用[105]。KOKUBU等[106]通过体外实验发现骨形态发生蛋白7的应用可以诱导神经膜细胞显著增生并提高其体外生存率,而应用甲状旁腺激素[parathyroidhormone,PTH(1-34)]可上调神经膜细胞骨形态发生蛋白7的水平,由此证明,损伤的周围神经损伤可引起去分化的神经膜细胞内骨形态发生蛋白/细胞内信号传导蛋白(BMP/Smad)信号通路的分子水平上调,以此调控神经的再生。此机制是通过调节机体内相关生物分子的表达来改变神经细胞膜的生理特性进而控制神经再生。这种通过机体回馈调节机制建立神经化的方向目前鲜有研究,这可以未来重建神经化的组织工程骨提供一个新的研究思路。
在骨骼发育和再生过程中,神经和血管向内生长在空间上是相互协调的,在功能上是相互依存的,实现神经血管耦合有助于改善骨骼重塑和代谢。神经血管耦合的机制是复杂的,各种生物活性物质及信号分子通道共同参与神经血管耦合。生物活性物质-血管-神经互相调控作用见图4。
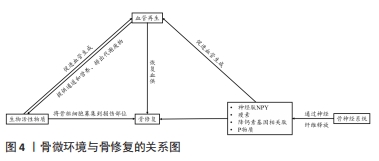
在骨缺损修复过程中,神经再支配后通常会发生血管化。相反,神经信号传导的抑制伴随着血管重建受阻和骨折愈合延迟[107]。感觉神经分泌血管内皮生长因子是影响血管生长和分化的主要因素[108]。此外,神经生长因子的表达可能在神经血管耦合中发挥关键作用,但其作用机制仍有待进一步研究。神经类物质对骨组织修复的影响见表3。

| [1] XU SF, YU XC, XU M, et al. Successful management of a childhood osteosarcoma with epiphysiolysis and distraction osteogenesis. Curr Oncol. 2014;21(4):e658-e662. [2] RAPOSO-AMARAL CE, BUENO DF, ALEMIDA AB, et al. Is bone transplantation the gold standard for repair of alveolar bone defects? J Tissue Eng. 2014;5: 2041731413519352. [3] GOODAM SB, PAJARINEN J, Yao Z, et al. Inflammation and bone repair: from particle disease to tissue regeneration. Front Bioeng Biotechnol. 2019;7:230. [4] HANKENSO KD, GAGNE K, SHAUGHNESSY M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3-12. [5] SCADDEN DT. The stem-cell niche as an entity of action. Nature. 2006;441 (7097):1075-1079. [6] DE JONG MME, KELLERMAYER Z, PAPAZIAN N, et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat Immunol. 2021;22(6):769-780. [7] 夏玉城,陶树清.低氧诱导因子和脯氨酸羟化酶在骨发育和骨稳态中的作用[J].实用骨科杂志,2020,26(4):339-342. [8] 谢玉,周诺.Ⅰ型胶原诱导骨髓间充质干细胞及成骨细胞的成骨分化机制[J].中国组织工程研究,2018,22(21):3417-3423. [9] MANCUSO P, RAMAN S, GLYNN A, et al. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol. 2019;7:9. [10] 许克惠,李娇娇,李香玉,等.光固化3D打印软组织材料的性能研究进展[J].中国生物医学工程学报,2019,38(5):628-635. [11] LAPNER P, BOULIANE M, POLLOCK JW, et al. Intraoperative channeling in arthroscopic rotator cuff repair: a multicenter randomized controlled trial. Am J Sports Med. 2023;51(2):323-330. [12] NAKANO K, MURATA K, OMOKAWA S, et al. Promotion of osteogenesis and angiogenesis in vascularized tissue-engineered bone using osteogenic matrix cell sheets. Plast Reconstr Surg. 2016;137(5):1476-1484. [13] CASANOVA MR, OLIVEIRA C, FERNANDES EM, et al. Spatial immobilization of endogenous growth factors to control vascularization in bone tissue engineering. Biomater Sci. 2020;8(9):2577-2589. [14] GREENHILL C. Bone. Formation of blood vessels in bone maturation and regeneration. Nat Rev Endocrinol. 2014;10(5):250. [15] FERRETTI C, RIPAMONTI U. Human segmental mandibular defects treated with naturally derived bone morphogenetic proteins. J Craniofac Surg 2002;13(3):434-444. [16] 武永刚,陈君长,王坤正.血管内皮细胞生长因子在骨折愈合过程中的表达[J].西安医科大学学报,2001,22(1):51-53, 61. [17] 张丽蓉,夏文杰,项鹏,等.体外定向诱导人骨髓间质干细胞分化为成骨细胞的研究[J].中国病理生理杂志,2002,18(7):745-748. [18] 丁生乐,孙正义.成纤维细胞生长因子对大鼠骨髓基质细胞诱导成骨的影响[J].第四军医大学学报,2003,24(2):119-122. [19] 彭磊,万明习,梁芳慧,等.骨髓成骨细胞在碱热处理的磷灰石涂层钛表面的分化增殖研究(英文)[J].稀有金属材料与工程,2004,33(10): 1018-1022. [20] 钟刚,裴福兴,樊瑜波,等.血管内皮生长因子基因转染促进成骨细胞的成骨活性(英文)[J].中国临床康复,2005,9(22):250-252. [21] 黄晓兵,刘霆,孟文彤,等.骨髓间充质干细胞分化的成骨细胞支持脐血造血干祖细胞的研究[J].中国实验血液学杂志,2006,14(3):552-556. [22] 刘勇,李小飞,程庆书,等.异体移植气管中骨形态发生蛋白诱导软骨再生的实验[J].中国临床康复,2006,10(1):91-93. [23] 智伟,邓力,杨志明,等.成骨细胞参与骨髓造血微环境的构建及发挥调控作用[J].中国修复重建外科杂志,2007,21(5):517-522. [24] ZHENG ZH, ZHU P, WANG YH, et al. In vitro induction of directional differentiation of bone marrow mesenchymal stem cells towards chondrocytes. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2005;21(1):79-82. [25] 李宁,吴桂英,李启明,等.不同氧浓度微环境对大鼠骨髓间充质干细胞成骨及成脂肪分化的影响[J].重庆医学,2009,38(19):2448-2450. [26] 穆晓红,赵子义,徐林,等.纳米材料支架与骨髓间充质干细胞构建纳米骨修复兔股骨头坏死[J].中国组织工程研究与临床康复,2010,14(51): 9582-9586. [27] 邓皖利,吴宏忠,徐文,等.阿胶补血活性组分对环磷酰胺所致贫血小鼠骨髓造血微环境的影响[J].时珍国医国药,2011,22(10):2542-2544. [28] 刘铁,苏庆军,藏磊,等.股骨头缺血性坏死与局部微环境瘦素表达的相关性研究[J].中国修复重建外科杂志,2012,26(11):1319-1323. [29] 陈玉玺,王晓莉,牟青杰,等.骨髓间充质干细胞对脑缺血大鼠星形胶质细胞影响的实验研究[J].医学研究生学报,2013,26(6):564-567. [30] 潘娅岚,马勇,郭杨,等.脊髓康对脊髓损伤大鼠脊髓组织结构及神经生长因子表达的影响[J].中国实验方剂学杂志,2014,20(15):144-149. [31] 习德娥,韩莉,谭超,等.P2X7受体与基因转录的关系[J].中国免疫学杂志,2015,31(9):1294-1296. [32] 钱海燕,陈慧敏,杜明亮,等.成骨细胞特异性识别多肽对人成骨细胞增殖和矿化影响的实验研究[J].安徽医科大学学报,2016,51(3):337-340. [33] 蒋欣泉.骨缺损修复生物材料与骨再生[J].中华口腔医学杂志,2017, 52(10):600-604. [34] HAUSHERR TC, NUSS K, THEIN E, et al. Effect of temporal onsets of mechanical loading on bone formation inside a tissue engineering scaffold combined with cell therapy. Bone Rep. 2018;8:173-179. [35] 刘国民,卢天成,冀璇,等.与胶原特异性结合的BMP2模拟肽/PLGA3D打印复合支架的制备及成骨诱导活性[J].高等学校化学学报, 2019,40(7):1552-1560. [36] LI F, Yu F, Liao X, et al. Efficacy of recombinant human BMP2 and PDGF-BB in orofacial bone regeneration: a systematic review and meta-analysis. Sci Rep. 2019;9(1):8073. [37] 赵士明,李文雷,赵静一,等.组织工程三维多孔骨支架内部微流体流场研究[J].高技术通讯,2020,30(5):518-525. [38] DOWLATSHAHI S, CHEN CY, ZIGDON-GILADI H, et al. Volumetric assessment of changes in the alveolar ridge dimension following guided bone regeneration using a combination freeze-dried bone allograft with collagen membrane or novel resorbable scaffold: a prospective two-center clinical trial. J Periodontol. 2022;93(3):343-353. [39] 王伟伟,欧志学,章晓云,等.外泌体在激素性股骨头坏死修复信号交流网络中的调控机制[J].中国组织工程研究,2022,26(19):3056-3064 [40] 冯韬,孟正华,郭巍.4D打印智能材料及产品应用研究进展[J].数字印刷,2022,218(3):1-16. [41] DELUCCHI Á, TORO L, ALZAMORA R, et al. Glucocorticoids decrease longitudinal bone growth in pediatric kidney transplant recipients by stimulating the FGF23/FGFR3 signaling pathway. J Bone Miner Res. 2019;34(10):1851-1861. [42] LI D, ZHAO D, ZENG Z, et al. Ternary regulation mechanism of Rhizoma drynariae total flavonoids on induced membrane formation and bone remodeling in Masquelet technique. PLoS One. 2022;17(12):e0278688. [43] SOBUE T, GRAVELY T, HAND A, et al. Regulation of fibroblast growth factor 2 and fibroblast growth factor receptors by transforming growth factor beta in human osteoblastic MG-63 cells. J Bone Miner Res. 2002;17(3):502-512. [44] TOOSI S, BEHRAVAN J. Osteogenesis and bone remodeling: a focus on growth factors and bioactive peptides. Biofactors. 2020;46(3):326-340. [45] 彭竑程,华臻,杨惠林,等.肌源性因子调控骨组织细胞的作用机制研究进展[J].中国修复重建外科杂志,2021,35(7):923-929. [46] KATAGIRI T, WATABE T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8(6):a021899. [47] EINHORN TA, GERSTENFELD LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [48] HU K, OLSEN BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016; 126(2):509-526. [49] HU K, OLSEN BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30-38. [50] SIDDIQUI JA, PARTRIDGE NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda). 2016; 31(3):233-245. [51] 何爱娟,张天宇.软骨组织工程种子细胞的研究进展[J].中国眼耳鼻喉科杂志,2020,20(1):3-6. [52] CHUNG JE, PARK JH, YUN JW, et al. Cultured human periosteum-derived cells can differentiate into osteoblasts in a perioxisome proliferator-activated receptor gamma-mediated fashion via bone morphogenetic protein signaling. Int J Med Sci. 2016;13(11):806-818. [53] 邢军超.间充质干细胞在组织工程骨修复骨缺损起始环节中的作用机理研究[D].重庆:第三军医大学,2014. [54] LIN X, PATIL S, GAO YG, et al. The bone extracellular matrix in bone formation and regeneration. Front Pharmacol. 2020;11:757. [55] 胡文成,朱弘一,林俊卿,等.细胞周基质介导骨关节炎发生发展的研究进展[J].上海交通大学学报(医学版),2021,41(8):1089. [56] ZHOU Z, LIU D. Mesenchymal stem cell-seeded porous tantalum-based biomaterial: a promising choice for promoting bone regeneration. Colloids Surf B Biointerfaces. 2022;215:112491. [57] 杨均,李澎.转化生长因子β诱导骨髓间充质干细胞分化为半月板纤维软骨细胞[J].中国组织工程研究,2023,27(15):2412-2419. [58] HO-SHUI-LING A, Bolander J, Rustom LE, et al. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143-162. [59] SAITO A, SUZUKI Y, OGATA S, et al. Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J Biomed Mater Res A. 2004;70(1): 115-121. [60] SAAD FA. Novel insights into the complex architecture of osteoporosis molecular genetics. Ann N Y Acad Sci. 2020;1462(1):37-52. [61] 吴穹. DKK1合成多肽对治疗骨质疏松症和成骨作用的研究[D].南京:南京大学,2011. [62] SARAN U, GEMINI PIPERNI S, CHATTERJEE S. Role of angiogenesis in bone repair. Arch Biochem Biophys. 2014;561:109-117. [63] HATTORI K, HEISSIG B, TASHIRO K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354-3360. [64] CHEN YC, LIN RZ, QI H, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22(10):2027-2039. [65] CORREIA C, GRAYSON WL, PARK M, et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One. 2011; 6(12):e28352. [66] GRELLIER M, FERREIRA-TOJAIS N, BOURGET C, et al. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem. 2009;106(3):390-398. [67] SAIK JE, GOULD DJ, WATKINS EM, et al. Covalently immobilized platelet-derived growth factor-BB promotes angiogenesis in biomimetic poly (ethylene glycol) hydrogels. Acta biomaterialia. 2011;7(1):133-143. [68] PATEL ZS, YOUNG S, TABATA Y, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931-940. [69] HATTORI K, HEISSIG B, TASHIRO K, et al. Plasma elevation of stromal cellderived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97(11):3354-3360. [70] PELTOLA MJ, AITASALO KM, SUONPAA JT, et al. In vivo model for frontal sinus and calvarial bone defect obliteration with bioactive glass S53P4 and hydroxyapatite. J Biomed Mater Res. 2001;58(3):261-269. [71] YOUNG S, PATEL ZS, KRETLOW JD, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15(9):2347-2362. [72] KAIGLER D, WANG Z, HORGERK, et al. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21(5):735-744. [73] ECKARDT H, DING M, LIND M, et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87(10):1434-1438. [74] PENG H, USAS A, OLSHANSKI A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017-2027. [75] 彭荟桢,蔡明详,刘湘宁.骨修复过程中的血管生成调控:新思路与新方法[J].中国组织工程研究,2022,26(15):2400-2405. [76] MOHYELDIN A, GARZON-MUVDI T, QUINONES-HINOJOSA A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7(2):150-161. [77] AMARILIO R, VIUKOV SV, SHARIR A, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134(21):3917-3928. [78] 张向荣.血管内皮细胞生长因子165基因转染人骨髓间充质干细胞构建组织工程皮肤的实验研究[D].南昌:南昌大学,2009. [79] CHEN X, HAN S, WU W, et al. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small. 2022;18(36):e2106824. [80] LUI YS, SOW WT, TAN LP, et al. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019;92:19-36. [81] LI YC, ZHANG YS, AKPEK A, et al. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1):012001. [82] 龙域丰,朱古力,易伟宏,等.神经肽类物质在骨代谢与骨再生中的调控作用[J].中华实验外科杂志,2020,37(11):2131-2136. [83] QIN Q, LEE S, PATEL N, et al. Neurovascular coupling in bone regeneration. Exp Mol Med. 2022;54(11):1844-1849. [84] CRAFT CS, SCHELLER EL. Evolution of the marrow adipose tissue microenvironment. Calcified tissue international. 2017;100(5):461-475. [85] 李俊琴,尹欣雨,张帅帅,等.感觉神经在骨修复中的作用及应用[J].生命科学,2020,32(3):227-232. [86] ZHANG Z, HAO Z, XIAN C, et al. Neuro-bone tissue engineering: multiple potential translational strategies between nerve and bone. Acta Biomaterialia. 2022;153:1-12. [87] HU CH, SUI BD, LIU J, et al. Sympathetic neurostress drives osteoblastic exosomal mir-21 transfer to disrupt bone homeostasis and promote osteopenia. Small Methods. 2022;6(3):e2100763. [88] YANG Y, ZHOU J, LIANG C, et al. Effects of highly selective sensory/motor nerve injury on bone metabolism and bone remodeling in rats. J Musculoskelet Neuronal Interact. 2022;22(4):524-535. [89] HU K, ZHOU H, ZHANG G, et al. The effect of chemical sympathectomy and stress on bone remodeling in adult rats. Neuro Endocrinol Lett. 2010; 31(6):807-813. [90] WANG T, CAO J, DU ZJ, et al. Effects of sympathetic innervation loss on mandibular distraction osteogenesis. J Craniofac Surg. 2012;23(5):1524-1528. [91] 邓宁,胡庆芬,邱宇阳,等. NGF介导Fabp4/LCN-2蛋白对骨质疏松大鼠骨微结构及骨强度的影响[J].中国骨质疏松杂志,2022,28(7):992-997. [92] TOMLINSON RE, LI Z, ZHANG Q, et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16(10):2723-2735. [93] ROSCH G, El BAGDADI K, MUSCHTER D, et al. Sympathectomy aggravates subchondral bone changes during osteoarthritis progression in mice without affecting cartilage degeneration or synovial inflammation. Osteoarthritis Cartilage. 2022;30(3):461-474. [94] RADDANT AC, RUSSO AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. [95] CHUNG AM. Calcitonin gene-related peptide (CGRP): role in peripheral nerve regeneration. Rev Neurosci. 2018;29(4):369-376. [96] 姚旺祥,马安,裴国献.神经肽在神经化组织工程骨中表达的早期实验研究[J].浙江创伤外科,2010,15(4):439-443. [97] SEIRADAKE E, JONES EY, KLEIN R. Structural perspectives on axon guidance. Annu Rev Cell Dev Biol. 2016;32:577-608. [98] KIM SK, PAK HN, PARK JH, et al. Cardiac cell therapy with mesenchymal stem cell induces cardiac nerve sprouting, angiogenesis, and reduced connexin43-positive gap junctions, but concomitant electrical pacing increases connexin43-positive gap junctions in canine heart. Cardiol Young. 2010;20(3):308-317. [99] MAPP PI, WALSH DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390-398. [100] 姚洋,杜宇,古霞,等.局部注射外源性神经生长因子促进小鼠钛种植体周骨胶原早期成熟的研究[J].华西口腔医学杂志,2018,36(2):128-132. [101] ELEFTERIOU F, AHN JD, TAKEDA S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032): 514-520. [102] YASUDA H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021;39(1):2-11. [103] MA F, LUO X, MA J, et al. The effect of the α7nAChR agonist on Wnt/β-catenin signaling in osteoporosis. Int J Clin Exp Pathol. 2019;12(8):2867-2874. [104] JIAO K, NIU LN, LI QH, et al. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci Rep. 2015;5(1):12593. [105] DAVIS EL, DAVIS AR, GUGALA Z, et al. Is heterotopic ossification getting nervous? The role of the peripheral nervous system in heterotopic ossification. Bone. 2018;109:22-27. [106] KOKUBU N, TSUJII M, AKEDA K, et al. BMP-7/Smad expression in dedifferentiated Schwann cells during axonal regeneration and upregulation of endogenous BMP-7 following administration of PTH (1-34). J Orthop Surg (Hong Kong). 2018;26(3):2309499018812953. [107] LI Z, MEYERS CA, CHANG L, et al. Fracture repair requires TrkA signaling by skeletal sensory nerves. J.Clin. Invest. 2019;129(12):5137-5150. [108] MUKOUYAMA YS, SHIN D, BRITSCH S, et al. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109(6):693-705. [109] NUKAVARAPU SP, DORCEMUS DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31(5):706-721. |
| [1] | 杨玉芳, 杨芷姗, 段棉棉, 刘毅恒, 唐正龙, 王 宇. 促红细胞生成素在骨组织工程中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1443-1449. |
| [2] | 陈凯佳, 刘景云, 曹 宁, 孙建波, 周 燕, 梅建国, 任 强. 组织工程技术在股骨头坏死治疗中的应用及前景[J]. 中国组织工程研究, 2024, 28(9): 1450-1456. |
| [3] | 王姗姗, 舒 晴, 田 峻. 物理因子促进干细胞的成骨分化[J]. 中国组织工程研究, 2024, 28(7): 1083-1090. |
| [4] | 张克凡, 石 辉. 细胞因子治疗骨关节炎的研究现状及应用前景[J]. 中国组织工程研究, 2024, 28(6): 961-967. |
| [5] | 王业元, 杜易朗, 于德浩, 宁凤婷, 白 冰. 微弧氧化处理对医用金属生物活性的影响[J]. 中国组织工程研究, 2024, 28(5): 771-776. |
| [6] | 王嘉旎, 陈俊宇. 金属离子促血管生成机制及在骨组织工程中的应用[J]. 中国组织工程研究, 2024, 28(5): 804-812. |
| [7] | 张 娅, 牟秋菊, 王自林, 刘宏杰, 祝丽丽. 负载富血小板血浆的水凝胶促进糖尿病大鼠创面愈合[J]. 中国组织工程研究, 2024, 28(5): 690-696. |
| [8] | 朱礼威, 王江玥, 白 丁. 纳米复合甲基丙烯酰明胶水凝胶在不同骨缺损环境中应用的价值[J]. 中国组织工程研究, 2024, 28(5): 753-758. |
| [9] | 朱小烽, 陈为玮, 黄 健. 母鼠高脂饮食与运动干预对雄性子代胰岛素敏感性及下丘脑弓状核的影响[J]. 中国组织工程研究, 2024, 28(4): 556-561. |
| [10] | 杨雨晴, 陈志宇. 早期短暂M1巨噬细胞在骨组织工程中的作用及应用[J]. 中国组织工程研究, 2024, 28(4): 594-601. |
| [11] | 买斯吐热木·黑力力, 张婉霞, 尼加提·努尔穆罕默德, 买买提吐逊·吐尔地. 关节腔注射医用臭氧对早期颞下颌骨关节炎模型大鼠髁突组织学的影响[J]. 中国组织工程研究, 2024, 28(4): 505-509. |
| [12] | 刘雪丽, 沈 丽, 毕文光, 牟 杨, 李 森. 低强度脉冲超声对大鼠急性肌腱损伤早期血管生成的影响及机制[J]. 中国组织工程研究, 2024, 28(32): 5097-5103. |
| [13] | 熊 洋, 周世博, 俞 兴, 毕连涌, 杨济洲, 王逢贤, 曲 弋, 杨永栋, 赵丁岩, 赵 赫, 仇子叶, 姜国正. 获得性异位骨化的分子生物学机制[J]. 中国组织工程研究, 2024, 28(30): 4881-4888. |
| [14] | 高雪钰, 张文涛, 孙天泽, 张 警, 李忠海. 金属离子在骨组织工程中的应用[J]. 中国组织工程研究, 2024, 28(3): 439-444. |
| [15] | 陈品叡, 裴锡波, 薛轶元. 磁响应水凝胶在骨组织工程中的作用与优势[J]. 中国组织工程研究, 2024, 28(3): 452-457. |
骨微环境是指能够近距离作用和调控骨组织细胞兴奋或抑制活动的微量理化因素或生物因素及骨组织细胞在分化过程中形成的、对相关细胞因子敏感的特殊结构和受体所组成的综合性功能环境[5-6]。骨微环境中骨质内的细胞主要包括骨细胞、破骨细胞及成骨细胞,其中成骨细胞和破骨细胞可以通过促进骨重塑来维护骨骼系统的完整[7]。而作为骨组织细胞赖以生存的局部条件,骨微环境能通过分泌与成骨分化相关的细胞因子参与骨髓间充质干细胞的增殖与分化活动[8]。细胞因子能在局部募集足量的骨髓间充质干细胞构建缺损部位的成骨微环境,诱导或者加快骨愈合过程[9]。改善骨组织微环境以促进骨整合是目前骨组织工程治疗的研究热点。
受损骨组织的修复是一个复杂的过程,适当的骨重塑需要一个积极的血管生成以及神经化过程,为骨重塑提供必要的生长因子和干细胞、神经递质以及各种激素。目前,骨组织工程常通过结合种子细胞、组织工程材料及生物化学因子等材料来调控骨组织微环境的变化,达到提升或替代受损骨组织器官的生物学功能的作用[10]。然而,种子细胞只有在血管周围150-200 μm的范围内才能通过营养的弥散得以存活,因此组织工程骨的早期血管化不足可能导致种子细胞因缺乏营养物质和氧气以及代谢产物过度积累而死亡[11-13]。此外,缺乏神经纤维的支配致使种子细胞缺少神经肽类物质的有效刺激,也是导致缺损部位成骨不足的重要原因[14]。充分的血液供应和完善的神经支配均是提高组织工程骨体内存活率的关键因素,故构建具有充足血供和神经支配的组织工程骨是骨组织工程由基础研究走向临床应用的关键一步。
文章通过对2022年12月前已发表的国内外相关文献进行检索并综合分析,从生物活性物质、血管生成及神经化3个视角对影响骨组织微环境的因素进行总结归纳。通过对国内外改变骨组织微环境以提高组织工程骨成骨效果的实验研究作介绍,并通过总结其观点及结论以期能为相关基础研究和临床治疗提供思路和参考。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
1.1.1 检索人及检索时间 由第一作者在2022年10-12月进行检索。
1.1.2 检索时限 检索各数据库 2001-2022 年的相关文献。
1.1.3 检索数据库 PubMed、Web of Science、Science Direct、万方及中国知网数据库。
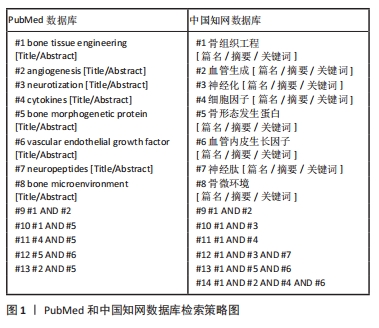
1.1.4 检索词 英 文 检 索 词 为“bone tissue engineering,angiogenesis,neurotization,cytokines,bone morphogenetic protein,vascular endothelial growth factor,neuropeptide,bone microenvironment”, 中文检索词为“骨组织工程,血管生成,神经化,细胞因子,骨形态发生蛋白,血管内皮生长因子,神经肽,骨微环境”。
1.1.5 检索文献类型 研究原著、综述、病例报告和临床研究。
1.1.6 手工检索情况 无。
1.1.7 检索策略 以PubMed和中国知网数据库检索策略为例,见图1。
1.2 入组标准
1.2.1 纳入标准 ①题目和摘要与主题词密切相关的文献;②与骨微环境、血管化和神经化联系紧密的文献;③证据等级足够并有实验数据支持的文献。
1.2.2 排除标准 ①与研究目的关联性不强的文献;②观点重复、陈旧的文章;③文献质量较差,数据支持不足的文章;④写作语言为中、英文以外语种的文献。
1.3 文献质量评估和数据的提取 对所有文献依据文献题目、摘要和关键词进行主题和质量的评估,剔除与文章目的无关、年代久远及重复的文献,共纳入英文文献72篇(PubMed数据库49篇,Web of Science数据库13篇,Science Direct数据库10篇),中文文献37篇(中国知网数据库24篇,万方数据库13篇),共109篇进行综述分析,见图2。
3.1.1 生物活性物质 细胞生长因子的能效是衡量组织工程材料是否具有生物活性及评估其修复能力的重要指标。负载细胞因子的组织工程支架曾被认为是指导组织再生最有效的策略,但由于多数细胞因子的半衰期较短且在常规给药过程中缺乏适当的载体,致使其在机体内弥散和降解速度过快而易丧失活性,将组织工程材料作为载体负载细胞因子能有效改善体内生物活性物质的呈递情况。由于细胞因子存在提取困难、不稳定且易失活等限制,人工合成具有细胞生长因子特性的生物活性物质是解决此问题的一种途径。
3.1.2 血管化 目前解决血管化问题的主要策略为细胞共培养、添加生长因子或种子细胞及设计个性化支架材料,选用多细胞因子差异性释放呈递系统能较好模拟体内细胞因子的供应模式,以调控骨损伤部位的微环境变化,实现血管的再生[109]。除释放种类和剂量外,还需探究血管生成相关生长因子的时效性及空间分布等诸多问题。目前应用组织工程骨治疗大体积骨缺损时仍存在较多困难,构建空间特异性结构调节血管诱导因子的空间排列以加速血管向缺氧区域生长可能是未来成功修复大面积骨缺损的有效方法。此外,随着近年来组织工程和基因工程的研究进展,利用转基因技术将血管生成调控因子基因导入种子细胞,能有效促进血管内皮细胞的某些特征或某些促血管化生长因子的表达。这一方法将成为未来重建组织工程骨血循环及修复大面积骨缺损的最具研究前景的治疗模式。
3.1.3 神经化 建立神经化后的组织工程骨能有效提高缺损部位神经肽的表达水平,对骨缺损部位起到良好的局部成骨效果。由神经系统释放的神经肽类物质通过与特定受体结合并作用于特定的信号通路,通过多种途径对骨生物学效应的关键性物质(包括细胞因子、神经生长因子、神经递质以及各种激素)进行调节,影响骨愈合、骨再生及成骨与破骨之间的平衡。而机体内相关生物分子的表达及活性变化可作用于神经细胞膜,使其生理特性改变,进而调控神经的再生。此外,在构建神经化的组织工程骨时,骨组织微环境变化与神经调控的作用是双向的,通过机体回馈调节机制建立神经化的方向目前鲜有研究,这可以未来重建神经化的组织工程骨提供一个新的研究思路。
3.2 既往他人在该领域研究的贡献和存在的问题 骨微环境保持稳态是骨骼细胞生长、转换、分化及维持正常功能的必要条件。骨组织所处的微环境较为复杂,多种信号分子和微环境理化性质都参与调控骨骼细胞与细胞、细胞与系统之间的交流,从多环节调控骨转换。骨微环境的紊乱不仅可能引起骨骼细胞成分改变,且可从多个环节导致骨转换失衡,进而导致骨质疏松及恶性肿瘤相关性骨病等多种疾病。尽管目前的研究在一定程度上揭示了骨微环境的影响因素,但针对骨微环境调控在组织工程中的综述多基于单一信号分子或微环境理化性质,其在模拟人体骨组织微环境成分以调控干细胞向骨组织再生的成骨谱系细胞的转化的研究与应用尚处于初级阶段,且相关综述对于神经系统对骨再生及重建的调控作用的总结概括还未完善,这限制了其在骨组织工程中的应用。另外,关于骨微环境变化影响缺损部位组织工程骨的治疗效果及确切的作用机制、多种细胞因子共同作用于血管再生以及通过机体回馈调节机制建立神经化的研究有待进一步探讨。
3.3 作者综述区别于他篇的特点 随着组织工程的迅速发展及在分子层面研究骨损伤修复机制不断深入,近几年出现大量生物活性物质、血管诱导因子、干细胞治疗及基因转导在骨组织工程中的应用,然而目前对于这些新兴的研究目前尚缺少详细的总结。目前针对骨微环境调控在组织工程中的综述多基于单一信号分子或微环境理化性质(包括酸碱度及温度等)。因此文章重点阐述了骨组织微环境中生物活性物质部分内在的分子联系,并归纳总结在血管化构建过程中需关注诱导分子在种类、剂量、作用时间以及空间分布等方面的问题。在构建血管化和神经化方面,发现骨形成的过程不是单一骨骼系统的参与调节,而是血液循环系统、神经系统和免疫系统等多系统同时参与、相互串联相互影响的过程。此外,文章分析认为骨组织微环境变化与神经调控的作用是双向的。
3.4 综述的局限性 文章对研究结果的表述不够深入,骨缺损修复再生的过程涉及多条成骨信号通路,关于骨微环境对干细胞向骨组织再生的成骨谱系细胞的转化的调控尚待进一步研究。而生物材料作为干细胞附着、生长和分化的三维框架,目前较常用于骨组织工程应用的生物活性材料主要包括无机材料、金属材料、有机材料和复合材料。生物材料在生物相容性和生物可降解性方面具有优势,不仅可以促进骨组织生长,同时也可避免意外的免疫反应。此外,骨形成过程和骨吸收过程之间的任何失衡都与各种炎症性骨疾病有关。骨与免疫系统之间的这些相互作用机制对于促进骨愈合、修复和提高再生的速度至关重要。但是目前实验研究多聚焦于生物活性物质的应用及血管重建,因此骨再生过程中涉及的免疫机制的研究及未纳入综述。同时由于生物材料相关研究为材料学领域研究内容,文章对此未作更进一步的分析。
3.5 综述的重要意义 以组织工程技术为基础的组织工程骨在引导骨再生技术上的应用有着巨大潜力。组织工程骨诱导骨组织重塑及再生的成功关键在于组织工程材料与体内缺损部位的局部骨骼微环境(生物活性物质、血管再生和神经重建)的融合程度以及对骨愈合过程中关键环节的调控。文章以骨组织缺损修复为切入点,系统性地阐述骨组织微环境各个组分的研究进展和其存在的分子联系,有助于在更加科学化的条件下如多因子共同作用或是在不同时间和空间的维度下进一步探究骨缺损修复的分子机制,为骨微环境的后续研究和应用提供参考依据,同时也为骨组织工程支架的研发和临床转化提供新的思路,具有重要意义。
3.6 课题专家组对未来的建议 骨组织工程的目的是将工程和生物特性结合起来,构建用于促进骨组织生长的临时人工环境。迄今为止,开发的主要策略包括生物活性支架、纳米医学、基于细胞的组合产品和3D打印生物陶瓷等。调节三维支架的特性可以有选择地引导细胞在骨组织中的发育和分化。骨组织工程的计算建模方法将是未来预测和优化细胞增殖和分化、再生组织生长、适应和维持方面极具潜力的临床应用工具。此外,计算模型可以帮助改善支架设计和高分辨率生物打印技术的使用,允许在微观和纳米尺度上进行细胞特异性设计。因此,正确理解细胞-生物材料相互作用的分子机制,将有利于实现在组织工程的功能性先进生物材料方面更深入的研究。未来的骨组织工程应该整合骨微环境、支架设计以及骨组织再生的模拟和预测等方面的基础知识,以多学科的视角进行骨缺损治疗的研究。 中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
 #br#
#br#
文题释义:
骨组织工程:通过结合种子细胞、组织工程材料及物理化学因子等要素来调控骨组织微环境的变化以诱导新的功能性骨再生,提升或替代受损骨组织器官的生物学功能。骨组织微环境:是指能够近距离作用和调控骨组织细胞兴奋或抑制活动的微量理化因素或生物因素及骨组织细胞在分化过程中形成的、对相关细胞因子敏感的特殊结构和受体所组成的综合性功能环境。
骨缺损主要是由创伤、感染、病理性骨折、肿瘤切除及骨髓炎清创等引起,尽管骨具有显著的再生能力,但骨缺损的再生和修复仍然是骨科手术的主要挑战。自体骨移植、异体骨移植和生物材料填充是目前治疗节段性骨缺损的主要方法,其中自体骨移植是公认修复骨损伤的“金标准”。但因自体骨量不足、供区二次损伤及移植骨吸收等问题限制了该治疗方法的应用。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||