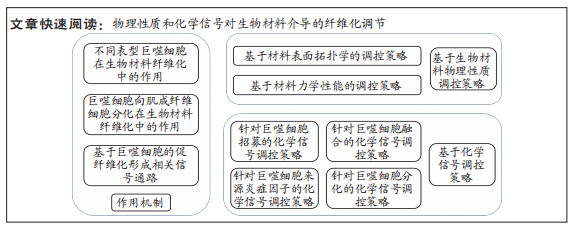
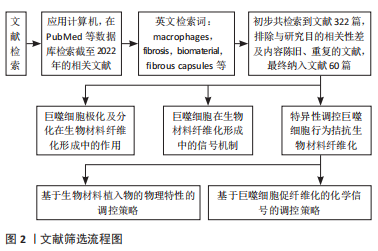
[1] WITHEREL CE, ABEBAYEHU D, BARKER TH, et al. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater. 2019;8(4):e1801451.
[2] NOSKOVICOVA N, HINZ B, PAKSHIR P. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells. 2021;10(7):1794.
[3] VASSE GF, NIZAMOGLU M, HEIJINK IH, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol. 2021;254(4):344-357.
[4] BORRIELLO F, LONGO M, SPINELLI R, et al. IL-3 synergises with basophil-derived IL-4 and IL-13 to promote the alternative activation of human monocytes. Eur J Immunol. 2015;45(7):2042-2051.
[5] WHEELER KC, JENA MK, PRADHAN BS, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. Plos One. 2018;13(1):e191040.
[6] HAIDER N, BOSCA L, ZANDBERGEN H R, et al. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J Am Coll Cardiol. 2019;74(25):3124-3135.
[7] HINZ B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64(4):171-177.
[8] NOSKOVICOVA N, SCHUSTER R, PUTTEN SV, et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-beta. Nat Biomed Eng. 2021;5(12):1437-1456.
[9] SCHUSTER R, ROCKEL JS, KAPOOR M, et al. The inflammatory speech of fibroblasts. Immunol Rev. 2021;302(1):126-146.
[10] DENG M, TAN J, HU C, et al. Modification of PLGA scaffold by MSC‐Derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF‐β‐induced protein. Advanced Healthcare Materials. 2020;13(9):2000353.
[11] DONDOSSOLAE, HOLZAPFEL BM, ALEXANDER S, et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2016;1:7.
[12] BANK RA, ZANDSTRA J, ROOM H, et al. Biomaterial encapsulation is enhanced in the early Stages of the foreign nody reaction during conditional macrophage depletion in transgenic macrophage fas-induced apoptosis mice. Tissue Eng Part A. 2017;23(19-20):1078-1087.
[13] WITHEREL CE, SAO K, BRISSON BK, et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269:120667.
[14] TAN RP, HALLAHAN N, KOSOBRODOVA E, et al. Bioactivation of encapsulation membranes reduces fibrosis and wnhances cell survival. ACS Appl Mater Interfaces. 2020;12(51):56908-56923.
[15] KAPS L, LEBER N, KLEFENZ A, et al. In Vivo siRNA Delivery to immunosuppressive liver macrophages by alpha mannosyl functionalized cationic nanohydrogel particles. Cells. 2020;9(8):1905.
[16] MCNALLY AK, ANDERSON JM. Phenotypic expression in human monocyte-derived interleukin-4-induced foreign body giant cells and macrophages in vitro: dependence on material surface properties. J Biomed Mater Res A. 2015;103(4):1380-1390.
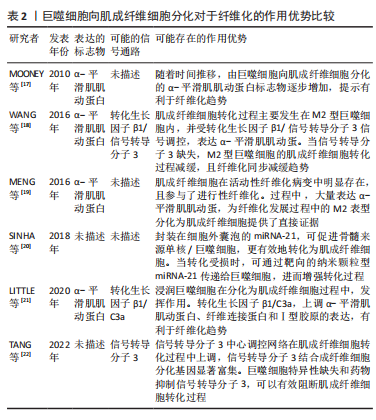
[17] MOONEY JE, ROLFE BE, OSBORNE GW, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol. 2010;176(1):369-380.
[18] WANG S, MENG XM, NG YY, et al. TGF-beta/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis. Oncotarget. 2016;7(8):8809-8822.
[19] MENG XM, WANG S, HUANG XR, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7(12):e2495.
[20] SINHA M, SEN C K, SINGH K, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nature Communications. 2018;9(1):936.
[21] LITTLE K, LLORIAN S M, TANG M, et al. Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration. J Neuroinflammation. 2020;17(1):355.
[22] TANG PCT, CHUNG JYF, XUE VWW, et al. Smad3 Promotes cancer associated fibroblasts generation via macrophage myofibroblast transition. Adv Sci. 2022;9(1):2101235.
[23] BEZHAEVA T, GEELHOED WJ, WANG D, et al. Contribution of bone marrow derived cells to in situ engineered tissue capsules in a rat model of chronic kidney disease. Biomaterials. 2019;194:47-56.
[24] CHAKRABORTY D, SUMOVA B, MALLANO T, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8(1):1130.
[25] KNIPPER JA, WILLENBORG S, BRINCKMANN J, et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity. 2015;43(4):803-816.
[26] FICHTNER FS, STROBER W, KAWAKAMI K, et al. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12(1):99-106.
[27] ZHANG B, ZHANG Z, XIA S, et al. KLF5 activates microRNA 200 transcription to maintain epithelial characteristics and prevent induced epithelial-mesenchymal transition in epithelial cells. Mol Cell Biol. 2013;33(24):4919-4935.
[28] BOWEN KA, DOAN HQ, ZHOU BP, et al. PTEN loss induces epithelial--mesenchymal transition in human colon cancer cells. Anticancer Res. 2009;29(11):4439-4449.
[29] LO CA, DELEVOYE C, GILLES MF, et al. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun. 2015;6:7506.
[30] KRICHEVSKY AM, GABRIELY G. MiR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39-53.
[31] WANG YY, JIANG H, PAN J, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol. 2017;28(7):2053-2067.
[32] TORRES A, MUNOZ K, NAHUELPAN Y, et al. Intraglomerular Monocyte/Macrophage Infiltration and Macrophage-Myofibroblast Transition during Diabetic Nephropathy Is Regulated by the A2B Adenosine Receptor. Cells. 2020;9(4):1051.
[33] WELLS RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013; 1832(7):884-890.
[34] VASSE GF, NIZAMOGLU M, HEIJINK IH, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol. 2021;254(4):344-357.
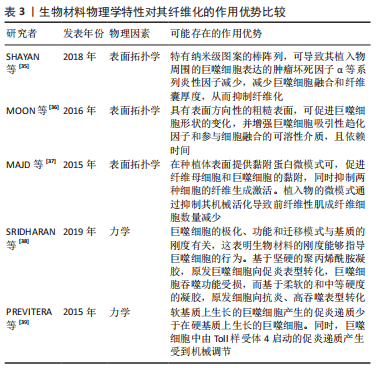
[35] SHAYAN M, PADMANABHAN J, MORRIS AH, et al. Nanopatterned bulk metallic glass-based biomaterials modulate macrophage polarization. Acta Biomater. 2018;75:427-438.
[36] MOON H, CREMMEL CV, KULPA A, et al. Novel grooved substrata stimulate macrophage fusion, CCL2 and MMP-9 secretion. J Biomed Mater Res A. 2016;104(9):2243-2254.
[37] MAJD H, SCHERER SS, BOO S, et al. Novel micropatterns mechanically control fibrotic reactions at the surface of silicone implants. Biomaterials. 2015;54:136-147.
[38] SRIDHARAN R, CAVANAGH B, CAMERON AR, et al. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47-59.
[39] PREVITERA ML, SENGUPTA A. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10(12):e145813.
[40] SCOTT RA, KIICK KL, AKINS RE. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 2021;122:220-235.
[41] WEI Q, HOLLE A, LI J, et al. BMP-2 Signaling and Mechanotransduction Synergize to Drive Osteogenic Differentiation via YAP/TAZ. Adv Sci (Weinh). 2020;7(15):1902931.
[42] KURODA M, UEDA K, KIOKA N. Vinexin family (SORBS) proteins regulate mechanotransduction in mesenchymal stem cells. Sci Rep. 2018;8(1):11581.
[43] DUPONT S, MORSUT L, ARAGONA M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179-183.
[44] MELI V S, ATCHA H, VEERASUBRAMANIAN PK, et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci Adv. 2020;6(49):eabb8471.
[45] FENG Y, LIANG Y, ZHU X, et al. The signaling protein Wnt5a promotes TGFbeta1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz. J Biol Chem. 2018; 293(50):19290-19302.
[46] BLAKNEY AK, SWARTZLANDER MD, BRYANT SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly (ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100(6):1375-1386.
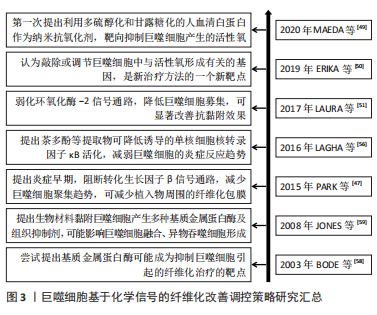
[47] PARK S, PARK M, KIM BH, et al. Acute suppression of TGF-ss with local, sustained release of tranilast against the formation of fibrous capsules around silicone implants. J Control Release. 2015;200:125-137.
[48] LIU S, PAN G, LIU G, et al. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release. 2017;264:1-13.
[49] MAEDA H, MINAYOSHI Y, ICHIMIZU S, et al. Repeated administration of Kupffer cells-targeting nanoantioxidant ameliorates liver fibrosis in an wxperimental mouse model. Biol Pharm Bull. 2020;43(1):93-101.
[50] ERIKA R, VLADIMIR R, DIEUWERTJE M M, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;2(224):242-253.
[51] LAURA F, FULVIO S, CRISTINA NA, et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep. 2017;19:1202-1213.
[52] HAN J, KIM YS, LIM MY, et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano. 2018;12(2):1959-1977.
[53] KAJAL C, SOUMYA K, MINJU J. Polygalactan from bivalve Crassostrea madrasensis attenuates nuclear factor-κB activation and cytokine production in lipopolysaccharide-activated macrophage. Carbohyd Polym. 2020;249:116817.
[54] MORRIS AH, MAHAL RS, UDELL J, et al. Multicompartment drug release system for dynamic modulation of tissue responses. Adv Healthc Mater. 2017. doi: 10.1002/adhm.201700370.
[55] ZHANG Y, ORISA JI. Exogenous oxidants activate nuclear factor kappa B through Toll-like receptor 4 stimulation to maintain inflammatory phenotype in macrophage. Biochem Pharmacol. 2018;147:104-118.
[56] LAGHA AB, GRENIER D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci Rep 2016;6:34520.
[57] YUE L, SHI YJ, SU XP, et al. Matrix metalloproteinases inhibitors in idiopathic pulmonary fibrosis: medicinal chemistry perspectives. Eur J Med Chem. 2021;224:113714.
[58] BODE W, MASKOS K. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol Chem Hoppe Seyler. 2003;384(6):863-872.
[59] JONES JA, MCNALLY AK, Chang DT, et al. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A. 2008;84(1):158-166.
[60] MENG XM , WANG S, HUANG XR, et al. Inflammatory macrophages can transdifferentiate into myofibroblasts during renal fibrosis. Cell Death Dis. 2016;7(12):e2495. |