中国组织工程研究 ›› 2023, Vol. 27 ›› Issue (3): 329-334.doi: 10.12307/2022.901
• 材料生物相容性 material biocompatibility • 下一篇
复合大孔聚多糖可吸收止血材料免疫毒性反应的评价
吴沥豪1,邵安良2,许 林1,任 康1,王洪建1,陈 亮2,许 零1
- 1厦门大学公共卫生学院,分子疫苗学与分子诊断学国家重点实验室,福建省厦门市 361102;2中国食品药品检定研究院,北京市 102629
Evaluation of immunotoxicity of the absorbable macroporous polysaccharides composite hemostatic material
Wu Lihao1, Shao Anliang2, Xu Lin1, Ren Kang1, Wang Hongjian1, Chen Liang2, Xu Ling1
- 1State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen 361102, Fujian Province, China; 2National Institutes for Food and Drug Control, Beijing 102629, China
摘要:
文题释义:
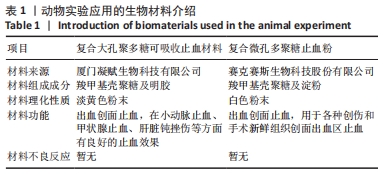
可吸收止血类医疗器械:指在常规止血技术无效的情况下,在手术过程中放置于人体内可被吸收的医疗器械产品,该类产品可通过加速创面局部血液的凝固过程产生止血作用。目前该类产品大多由以下材质制成:氧化纤维素、再生氧化纤维素、淀粉等植物多糖,以及可吸收性明胶、胶原等。该文的研究对象为以羧甲基壳聚糖和明胶为主要原材料的新型可吸收止血材料。
免疫毒性实验:免疫毒理学实验是观察药物对实验动物免疫系统产生不良影响的机制。免疫毒性研究已经作为药物非临床安全性评价的一项重要内容,在全面评价药物毒性方面发挥着越来越重要的作用。
背景:研究表明复合大孔聚多糖可吸收止血材料具有良好的止血效果,但目前关于其免疫毒性未进行深入研究。
目的:评价复合大孔聚多糖可吸收止血材料在小鼠体内的免疫毒性。
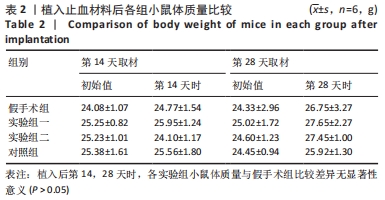
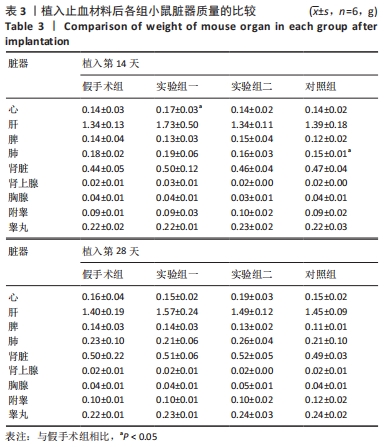
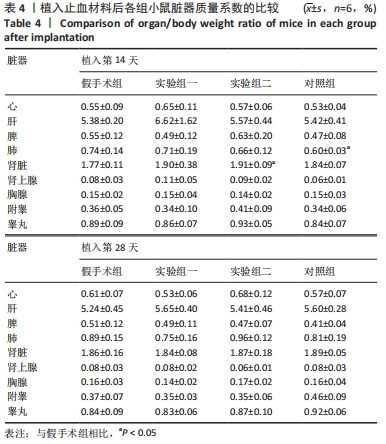
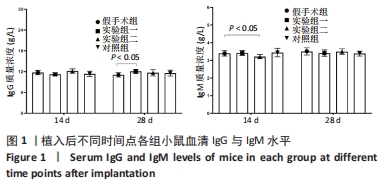
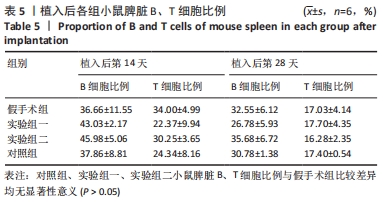
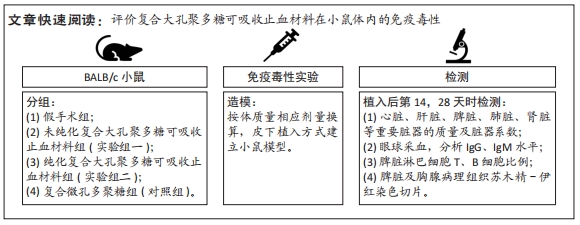
方法:将48只BALB/c小鼠随机分为4组,每组12只,假手术组皮下囊不植入材料,实验组一皮下囊植入未纯化复合大孔聚多糖可吸收止血材料,实验组二皮下囊植入纯化复合大孔聚多糖可吸收止血材料,对照组皮下囊植入复合微孔多聚糖止血粉。植入后第14,28天时,分析小鼠心脏、肝脏、脾脏、肺脏、肾脏、肾上腺、胸腺、睾丸、附睾的质量及脏器质量系数,血清IgG、IgM浓度,脾脏淋巴细胞中T、B细胞比例,脾脏及胸腺病理组织变化。
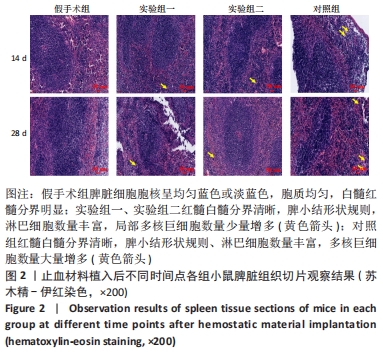
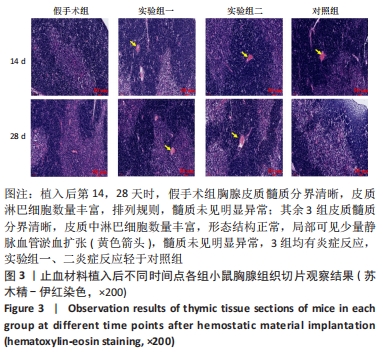
结果与结论:①实验过程中,植入材料小鼠状态良好,无明显中毒反应;②植入后第14天时,实验组一心脏质量高于假手术组(P < 0.05),对照组肺脏质量及质量系数低于假手术组(P < 0.05),实验组二肾脏质量系数高于假手术组(P < 0.05);植入后第14,28天,植入材料3组其余脏器质量及脏器质量系数与假手术组比较差异均无显著性意义(P > 0.05);③实验组二植入后第14天的IgM浓度低于假手术组(P < 0.05),实验组一植入后第28天的IgG浓度高于假手术组(P < 0.05);④植入后第14,28天,实验组一、实验组二、对照组B、T淋巴细胞比例与假手术组比较差异均无显著性意义(P > 0.05);⑤植入后第14,28天的苏木精-伊红染色显示,植入材料3组均有轻微慢性炎症,炎性反应程度为实验组二<实验组一<对照组;⑥结果表明,复合大孔聚多糖可吸收止血材料具有较低的免疫毒性。
https://orcid.org/0000-0003-0578-6914 (吴沥豪)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料;口腔生物材料;纳米材料;缓释材料;材料相容性;组织工程
中图分类号: