中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (34): 5448-5453.doi: 10.12307/2022.454
• 材料生物相容性 material biocompatibility • 上一篇 下一篇
注射用聚左旋乳酸微球体内可促胶原再生
张译心1,罗 倩2,梁瀚文2,陈建林2,赵 楠3,何 斌4
- 1重庆电子工程职业学院智慧健康学院,重庆市 401331;2成都医学院检验医学院,四川省成都市 610500;3普丽妍(南京)医疗科技有限公司,江苏省南京市 211500;4四川大学国家生物医学材料工程技术研究中心,四川省成都市 610064
In vivo neocollagenesis of injectable poly(L-lactic acid) microspheres
Zhang Yixin1, Luo Qian2, Liang Hanwen2, Chen Jianlin2, Zhao Nan3, He Bin4
- 1School of Smart Health, Chongqing College of Electronic Engineering, Chongqing 401331, China; 2School of Laboratory Medicine, Chengdu Medical College, Chengdu 610500, Sichuan Province, China; 3Puliyan (Nanjin) Medical Technology Co., Ltd., Nanjing 211500, Jiangsu Province, China; 4National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, Sichuan Province, China
摘要:

文题释义:
微球:粒径在1-1 000 μm范围内的球形粒子。不同于胶束等自组装体系中粒径大小和形貌由材料的性质和自组装驱动力决定,微球的粒径和形貌可通过控制制备参数进行调控,以符合不同应用的需求。
胶原再生:胶原蛋白(简称胶原)是皮肤的重要组成成分,主要起支撑、弹性的作用。随着年龄增长或外界环境(紫外线)的影响,皮下软组织缺失而导致了皱纹、褶皱的产生。通过皮下注射聚左旋乳酸微球刺激机体产生异物反应,从而形成由胶原组成的纤维囊,以达到面部年轻化的目的。
背景:聚左旋乳酸皮下填充剂是美国FDA批准可作为修复皮下软组织胶原流失的医美产品,由于其形态为不规则颗粒,容易产生过度炎症反应。
目的:观察辐照条件对聚左旋乳酸微球分子质量和粒径形貌的影响,以及聚左旋乳酸微球皮下填充剂植入兔皮下的异物反应程度和刺激胶原再生情况。
方法:采用乳液-溶剂挥发法制备聚左旋乳酸微球,参考Sculptra®的配方配制成可注射皮下填充剂,分别经25,50 kGy辐照灭菌,分析辐照灭菌对微球分子质量和粒径形貌的影响。采用MTT法检测不同质量浓度(50,100,250,500 mg/L,以微球的浓度计)可注射皮下填充剂对小鼠成纤维细胞增殖的影响。将25 kGy辐照灭菌的可注射皮下填充剂(实验组)、生理盐水(对照组)分别注射至兔背部皮下,于设定的时间点进行注射部位背部组织苏木精-伊红、马松三色染色及CD68、Ⅰ型胶原和Ⅲ型胶原免疫荧光染色。
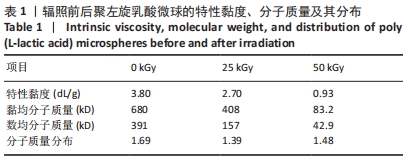
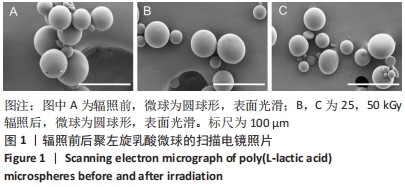
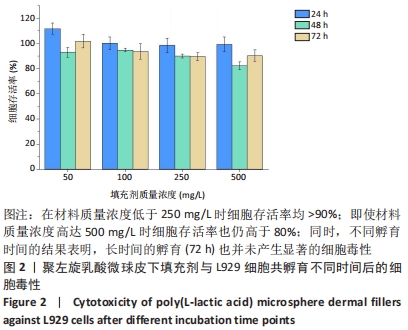
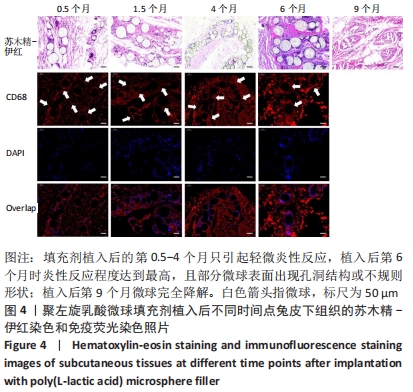
结果与结论:①随着辐照剂量的增加,微球的黏度、黏均分子质量和数均分子质量降低明显,但辐照灭菌并未导致微球的粒径和形貌发生明显改变。②MTT检测结果显示,当填充剂的质量浓度低于250 mg/L时细胞存活率均>90%,即使材料质量浓度高达500 mg/L时细胞存活率也仍高于80%,并且长时间的孵育(72 h)也并未产生显著的细胞毒性。③动物实验苏木精-伊红染色与CD68免疫荧光染色显示,填充剂植入后的第0.5-4个月只引起轻微炎性反应,植入后第6个月时炎性反应程度达到最高,且部分微球表面出现孔洞结构或不规则形状;植入后第9个月微球完全降解。④动物实验马松三色染色与Ⅰ型胶原、Ⅲ型胶原免疫荧光染色显示,植入后第4个月,微球周围主要为Ⅰ型胶原,外围主要以Ⅲ型胶原为主;植入后第6个月,微球附近的I型胶原增多,外围的Ⅲ型胶原增多;植入后第9个月,纤维包囊内部主要以Ⅰ型胶原为主,外围则Ⅰ型胶原与Ⅲ型胶原的比例接近均等。⑤结果表明,可注射聚左旋乳酸微球填充剂具有刺激胶原再生的效果,还可降低炎性反应程度。
https://orcid.org/0000-0003-2414-4730 (张译心)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料; 口腔生物材料; 纳米材料; 缓释材料; 材料相容性;组织工程
中图分类号: