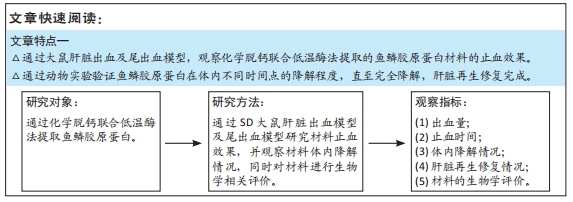
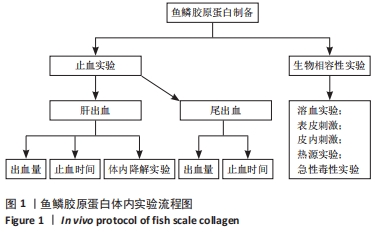
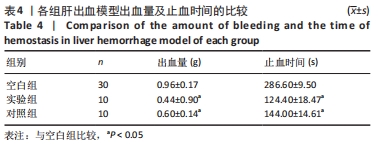
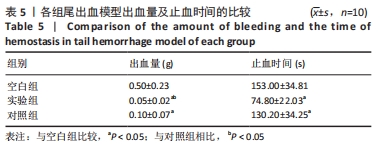
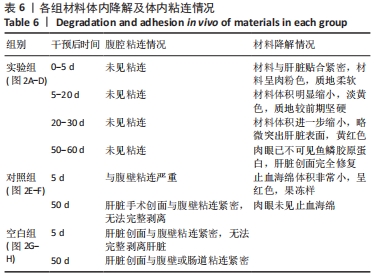
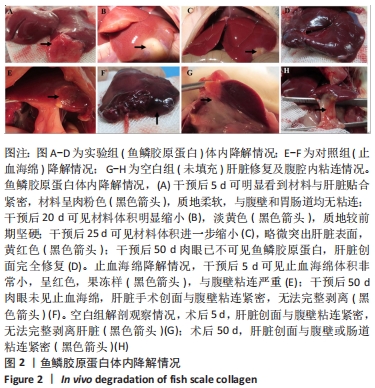
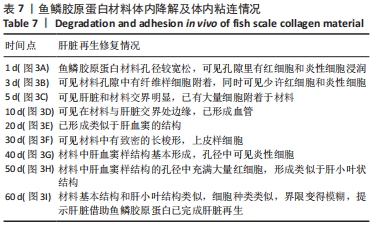
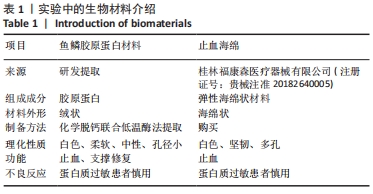
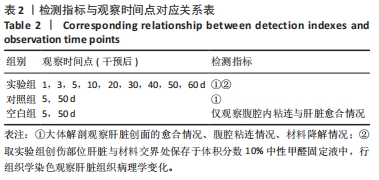
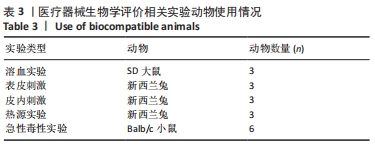
[1] 杨珏莹,林礼智,陈煜,等.急救用新型快速止血材料研究进展[J].化工新型材料,2020,48(7):24-29.
[2] 张爽,徐庆华,童琳,等.可吸收止血材料的研究现状与应用[J].中国组织工程研究,2021,25(10):1628-1634.
[3] 张爽,叶红,童琳,等.可吸收止血材料效果评价方法[J].中国医药生物技术,2019,14(6):561-563.
[4] 邓乐君,樊鸿浩,李伟达,等.可吸收止血材料的生物相容性研究进展[J].中国生物医学工程学报,2016,35(2):241-246.
[5] 柳小军,徐玉茵,周静,等.可溶性止血纱布对兔肝脏创伤的止血作用[J].中国组织工程研究,2016,20(47):7070-7075.
[6] HICKMAN DA, PAWLOWSKI CL, SEKHON U, et al. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv Mater. 2018;30(4)1-40.
[7] 陈星陶,李漱阳,熊熠,等.介孔生物玻璃微球/羧甲基纤维素复合可吸收止血材料[J].高分子材料科学与工程,2020,36(6):118-123.
[8] CHENG X, SHAO Z, LI C, et al. Isolation, Characterization and evaluation of collagen from jellyfish rhopilema esculentum kishinouye for use in hemostatic applications. PLoS One. 2017;12(1):e169731.
[9] WU S, APPLEWHITE AJ, NIEZGODA J, et al. Oxidized regenerated cellulose/collagen dressings: review of evidence and recommendations. Adv Skin Wound Care. 2017;30 (11S Suppl 1):S1-S18.
[10] CHIARA O, CIMBANASSI S, BELLANOVA G, et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018;18(1):68.
[11] 王璐璐,王少华.局部可吸收止血材料的应用现状及其研究进展[J].医学研究生学报,2018,31(1):109-112.
[12] MENDIS E, RAJAPAKSE N, BYUN HG, et al. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005;77(17):2166-2178.
[13] NGO DH, RYU B, VO TS, et al. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int J Biol Macromol. 2011;49(5):1110-1116.
[14] HIMAYA S, NGO DH, RYU BM, et al. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chemistry. 2012;132(4):1872-1882.
[15] MARTINEZ-ALVAREZ O, ALEMAN A. Marine collagen as a source of bioactive molecules. A review. Natural Products J. 2013;3(2):105-114.
[16] GUAMBE JF, MARS JA, DAY J. Application of PIXE in pollution control of the Matola River in Mozambique-analysis of fish scales. Nucl Instrum Methods Phys Res. 2012;273:171-172.
[17] 冯文婕,赵粼,阙斐.酸酶复合法优化鱼鳞胶原蛋白的提取工艺[J].湖北农业科学,2016,55(5):1242-1246.
[18] 中华人民共和国国家质量监督检验检疫总局、中国国家标准化管理委员会. 医疗器械生物学评价 第1部分:风险管理过程中的评价与实验:GB/T 16886.1-2011[S]. 2011.
[19] MEHRZAD AS, MONICAN. Effect of fibrin packing on managing hepatic hemorrhage and liver wound healing in a model of liver stab wound in rat. Bull Emerg Trauma. 2018;5(1):18-23.
[20] GEDAR OM, GUZEL SE, EKICI H, et al. Effects of algan hemostatic agent on bleeding time in a rat tail hemorrhage model. Ulus Travma Acil Cerrahi Derg. 2020;26(6):853-858.
[21] 中华人民共和国国家质量监督检验检疫总局、中国国家标准化管理委员会. 医疗器械生物学评价 第4部分:与血液相互作用实验选择:GB/T 16886.4-2003[S]. 2003.
[22] 中华人民共和国国家质量监督检验检疫总局、中国国家标准化管理委员会. 医疗器械生物学评价第10部分:刺激与皮肤致敏实验:GB/T 16886.10-2017[S]. 2017.
[23] 中华人民共和国国家质量监督检验检疫总局、中国国家标准化管理委员会. 医疗器械生物学评价 第11部分:全身毒性实验:GB/T 16886.11-2011[S]. 2011.
[24] 李保强,王利强,丁建虹,等.鱼鳞胶原蛋白的研究进展[J].包装工程,2018,39(17): 53-60.
[25] YAN T, CHENG F, WEI X, et al. Biodegradable collagen sponge reinforced with chitosan/calcium pyrophosphate nanoflowers for rapid hemostasis. Carbohydr Polym. 2017;170:271-280.
[26] 赵然,曹敏杰,王晶,等.水产动物源胶原蛋白的提取及应用研究进展[J].食品安全质量检测学报,2020,11(22):8157-8165.
[27] GU R, SUN W, ZHOU H, et al. The performance of a fly-larva shell-derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials. 2010;31(6):1270-1277.
[28] 田靖,崔庆华,郭平,等.小鼠剪尾出血模型的优化[J].实验动物与比较医学,2017,37(1):40-45.
[29] PISMENSKY SV, KALZHANOV ZR, ELISEEVA MY, et al. Severe inflammatory reaction induced by peritoneal trauma is the key driving mechanism of postoperative adhesion formation. BMC Surg. 2011;11:30.
[30] SLUITER N, DE CE, KWAKMAN R, et al. Adhesion molecules in peritoneal dissemination: function, prognostic relevance and therapeutic options. Clin Exp Metastasis. 2016;33(5):401-416.
[31] SUAREZ-GRAU JM, RUBIO CC, MORALES-CONDE S, et al. Could we reduce adhesions to the intra-abdominal mesh in the first week? Experimental study with different methods of fixation. Hernia. 2020; 24(6):1245-1251.
[32] JIANG X, WANG Y, FAN D, et al. A novel human-like collagen hemostatic sponge with uniform morphology, good biodegradability and biocompatibility. J Biomater Appl. 2017;31(8):1099-1107.
[33] SUN L, LI B, JIANG D, et al. Nile tilapia skin collagen sponge modified with chemical cross-linkers as a biomedical hemostatic material. Colloids Surf B Biointerfaces. 2017;159:89-96.
[34] 石长灿,赵瑾,刘雯,等.可吸收止血材料的研究与应用进展[J].高分子通报,2018(5):1-13.
[35] XIE H, CHEN X, SHEN X, et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int J Biol Macromol. 2018;107(Pt A):93-104.
[36] 刘雨萱,陈媛,李美良,等.罗非鱼鱼鳞胶原蛋白的研究进展[J].食品工业科技,2019,40(6):355-360.
|