中国组织工程研究 ›› 2022, Vol. 26 ›› Issue (4): 528-534.doi: 10.12307/2022.087
• 药物控释材料 drug delivery materials • 上一篇 下一篇
盐酸万古霉素@聚乳酸-羟基乙酸共聚物-壳聚糖-透明质酸复合缓释微球的制备及体外评价
乐国平,张 明,席立成,罗瀚文
- 广西医科大学第四附属医院骨病关节外科/骨肿瘤科,广西壮族自治区柳州市 545000
Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres
Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen
- Department of Bone Disease and Joint Surgery/Bone Oncology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou 545000, Guangxi Zhuang Autonomous Region, China
摘要: 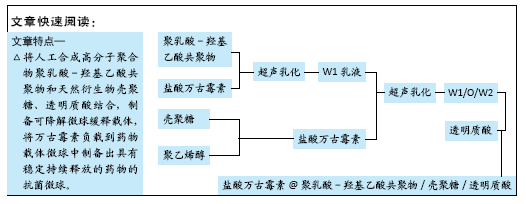
文题释义:
缓释微球:是用特殊的材料将药物负载其中制成的微球制剂,药物通过微球表面的微孔缓慢释放,避免药物在短时间的迅速释放,延长药物吸收和分布及作用时间,减少血药浓度忽高忽低带来的毒副作用。
药物缓释系统:是将药物负载于某种材料上直接作用于病变组织处,使得药物呈持续缓慢释放的一种新的给药系统,这种治疗模式的成功主要是在感染局部提供了可持续的有效杀菌浓度,同时避免了因为全身性抗生素治疗带来的药物毒性。
背景:局部抗生素缓释系统可解决全身应用抗生素时引发的全毒性反应与短期局部注射抗生素半衰期短的问题。
目的:制备盐酸万古霉素@聚乳酸-羟基乙酸共聚物-壳聚糖-透明质酸[vaneomyeinhydroehlorid@poly(lactic acid glycolic acid)-chitosan-hyaluronic acid,VA@PLGA-CS-HA]复合缓释微球,并对其性能进行评价。
方法:采用乳液法制备VA@PLGA-CS-HA复合缓释微球与未载药PLGA-CS-HA复合微球,其中载药微球中万古霉素的质量浓度分别为25,50,100 g/L,检测载药微球的载药量、包封率与体外缓释性能。将3种载药微球分别与金黄色葡萄球菌菌液共培养,相应时间点内检测抑菌率。将4种微球浸提液分别与MC3T3-E1细胞和MG-63细胞共培养,培养1,3,7 d后采用CCK-8法检测细胞毒性。
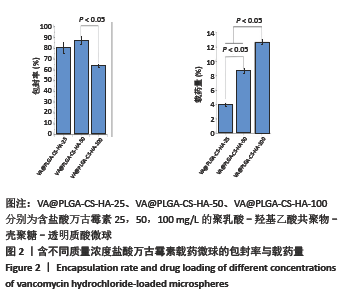
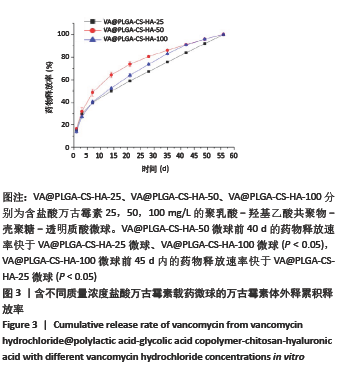
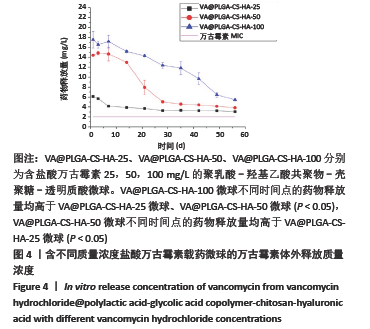
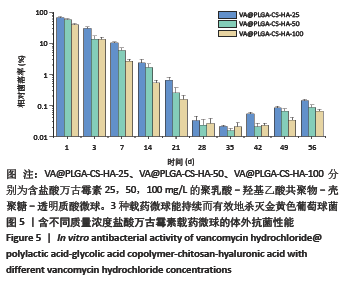
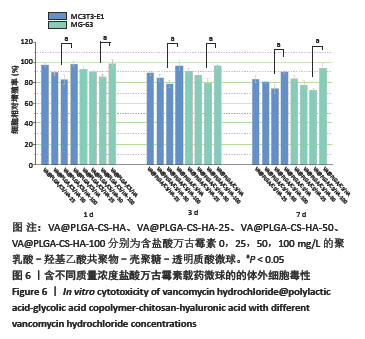
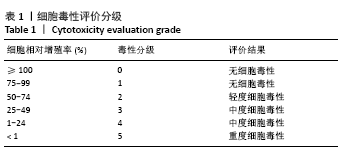
结果与结论:①含盐酸万古霉素25,50,100 g/L载药微球的包封率分别为(79.70±5.11)%,(86.41±3.91)%,(63.18±1.96)%,载药量分别为(3.98±0.26)%,(8.64±0.39)%,(12.63±0.39)%;50 g/L载药微球的包封率高于100 g/L载药微球(P < 0.05),100 g/L载药微球的载药量高于其他两组(P < 0.05);②3种载药微球在24 h内均无明显的突释,其中50 g/L载药微球不同时间点的药物释放率快于其他两组,100 g/L载药微球不同时间点的药物释放量高于其他两组,并且3组在56 d时释放的药物质量浓度均高于盐酸万古霉素最小抗菌浓度;③3种载药微球均能在一定时间内有效杀死金黄色葡萄球菌,在第14-28天期间3种微球的相对菌落率低于3%,说明3种载药微球能持续而有效杀灭金黄色葡萄球菌;④含盐酸万古霉素25,50 g/L载药微球对MC3T3-E1细胞和MG-63细胞无明显的细胞毒性,100 g/L载药微球具有一定的细胞毒性;⑤结果表明,VA@PLGA-CS-HA微球具有良好的缓释性能、抗菌能力与生物组织相容性。
https://orcid.org/0000-0001-7002-599X (乐国平)
中国组织工程研究杂志出版内容重点:生物材料;骨生物材料; 口腔生物材料; 纳米材料; 缓释材料; 材料相容性;组织工程
中图分类号: