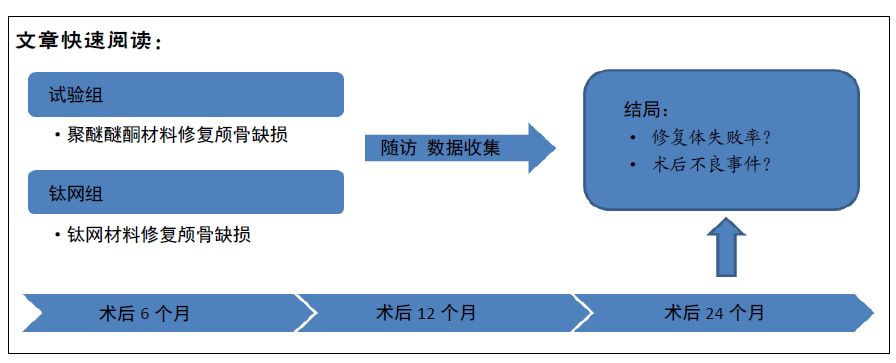
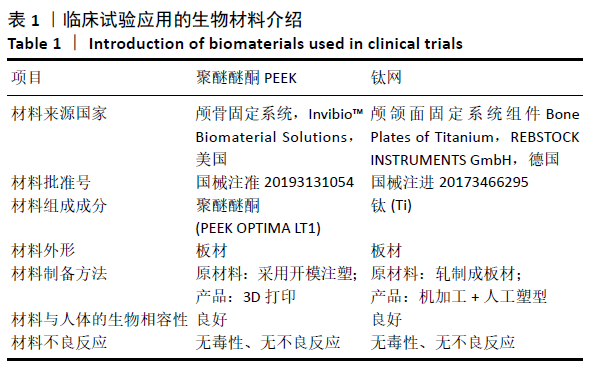
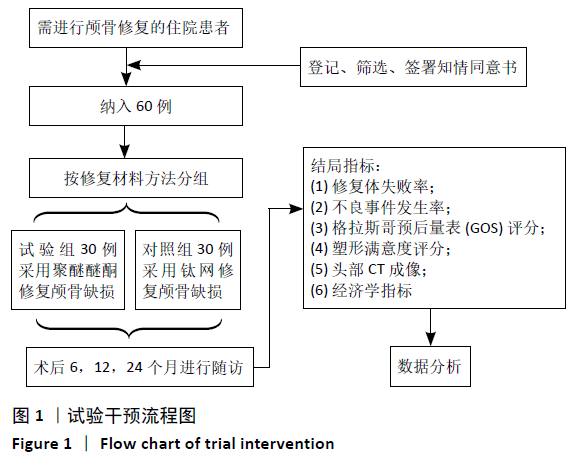
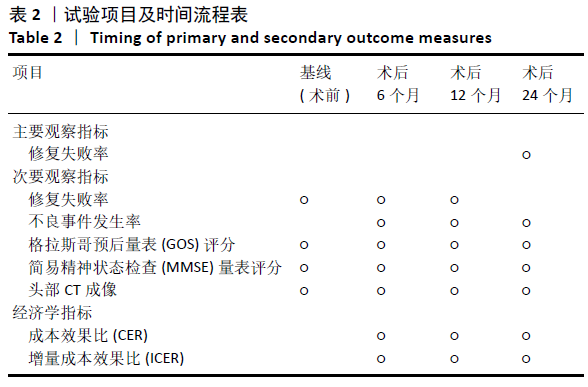
[1] 刘百雨,王忠.颅骨成形术的研究进展[J].中国医药导报,2020,17(7):35-38.
[2] 向迅捷,黄华,徐才邦,等.颅骨成形术后脑组织塌陷区压力变化规律[J].中国临床神经外科杂志,2020,25(10):707-709.
[3] 邢海涛,袁波,谭占国.同期与分期行脑室-腹腔分流术与颅骨成形术治疗颅脑损伤术后脑积水的疗效分析[J].中国临床神经外科杂志,2020,25(11):750-752.
[4] ZHANG J, TIAN W, CHEN J, et al. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res Bull. 2019;153:143-149.
[5] 陈智谦,穆雄铮.颅骨缺损修补材料应用的Meta分析[J].中国组织工程研究, 2018,22(30):4913-4920.
[6] 孙新林,王继辉,黄敏,等.聚醚醚酮与钛网在去骨瓣减压术后颅骨成形术中临床应用效果的单中心回顾性研究[J].中华神经医学杂志,2018,17(8):825-830.
[7] 孙玉晨,出良钊,董明昊,等.聚醚醚酮与钛网在颅骨成形术中应用体会[J].中国临床神经外科杂志,2018,23(2):103-104.
[8] OLIVER JD, BANUELOS J, ABU-GHNAME A, et al. Alloplastic cranioplasty reconstruction: a systematic review comparing outcomes with titanium mesh, polymethyl methacrylate, polyether ether ketone, and norian implants in 3591 adult patients. Ann Plast Surg. 2019;82(5S Suppl 4):S289-S294.
[9] Punchak M, Chung LK, Lagman C, et al. Outcomes following polyetheretherketone (PEEK) cranioplasty: systematic review and meta-analysis. J Clin Neurosci. 2017;41:30-35.
[10] 林柳兰,周建勇.3D打印聚醚醚酮及其复合材料修复骨缺损的应用现况[J].中国组织工程研究,2020,24(10):1622-1628.
[11] 洪咏龙,何海涛,隋文,等.个体化聚醚醚酮植入体修复颅颌面骨缺损[J].中华神经外科疾病研究杂志,2016,15(3):274-276.
[12] 乔梁,王汉东,宋玥,等.应用计算机辅助设计和制作的聚醚醚酮修补材料行颅骨成形术的临床观察[J].创伤外科杂志,2019,21(11):805-807.
[13] 张国斌,张述升,靳峥,等.以聚醚醚酮植入体行颅骨缺损成形术的初步研究[J].天津医药,2017,45(8):806-809,封2.
[14] 夏明,邵珠平,李文化,等.三维钛网颅骨修补术区感染原因分析[J].中国临床医生杂志,2020,48(1):74-76.
[15] 张勇,葛玉元,余万.数字化塑形钛网颅骨缺损修补术后并发症分析[J].江苏医药,2018,44(11):1339-1341.
[16] JONKERGOUW J, VAN DE VIJFEIJKEN SE, NOUT E, et al. Outcome in patient-specific PEEK cranioplasty: a two-center cohort study of 40 implants. J Craniomaxillofac Surg. 2016;44(9):1266-1272.
[17] LETHAUS B, SAFI Y, TER LAAK-POORT M, et al. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J Neurotrauma. 2012; 29(6):1077-1083.
[18] NG ZY, ANG WJ, NAWAZ I. Computer-designed polyetheretherketone implants versus titanium mesh (± acrylic cement) in alloplastic cranioplasty: a retrospective single-surgeon, single-center study. J Craniofac Surg. 2014;25(2):e185-e189.
[19] THIEN A, KING NK, ANG BT, et al. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2015; 83(2):176-180.
[20] YANG J, SUN T, YUAN Y, et al. Evaluation of titanium mesh cranioplasty and polyetheretherketone cranioplasty: protocol for a multicentre, assessor-blinded, randomised controlled trial. BMJ Open. 2019;9(12):e033997.
[21] YANG J, SUN T, YUAN Y, et al. Evaluation of titanium cranioplasty and polyetheretherketone cranioplasty after decompressive craniectomy for traumatic brain injury: a prospective, multicenter, non-randomized controlled trial. Medicine (Baltimore). 2020;99(30):e21251.
[22] 乌拉别克·毛力提,杜伟,刘伟,等.脑室-腹腔分流术及颅骨修补术同期治疗颅脑损伤去骨瓣减压术后并交通性脑积水的有效性[J].中国临床保健杂志,2020,23(5):668-671.
[23] 龙宇波.颅脑外伤去骨瓣减压术后早期行颅骨修补术的临床价值研究[J].中国医药导刊,2018,20(8):457-460.
[24] 李深誉.早期颅骨修补术对颅骨缺损患者脑灌注、神经功能、认知功能和生活质量的影响[J].神经损伤与功能重建,2017,12(6):567-568.
[25] 郑新瑞,胡世颉,闫志强,等.硬膜双层修补在额颞区大骨瓣减压术后颅骨缺损修补中的临床效果研究[J].中国医学前沿杂志(电子版),2017,9(10):62-66.
[26] ZHANG Q, YUAN Y, LI X, et al. A large multicenter retrospective research on embedded cranioplasty and covered cranioplasty. World Neurosurg. 2018;112: e645-e651.
[27] Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367:1355-1360.
[28] HANASONO MM, GOEL N, DEMONTE F. Calvarial reconstruction with polyetheretherketone implants. Ann Plast Surg. 2009;62:653-655.
[29] CAMARINI ET, TOMEH JK, DIAS RR, et al. Reconstruction of frontal bone using specific implant polyether-ether-ketone. J Craniofac Surg. 2011;22:2205-2207.
[30] 乔梁,王汉东,宋玥,等.应用计算机辅助设计和制作的聚醚醚酮修补材料行颅骨成形术的临床观察[J].创伤外科杂志, 2019,21(11):805-807. |