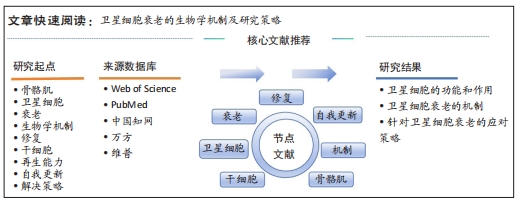
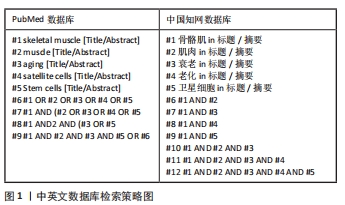
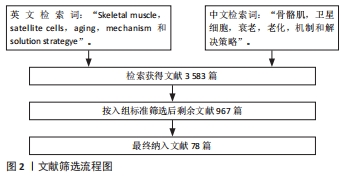
[1] SCHMIDT M, SCHÜLER SC, HÜTTNER SS, et al. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci. 2019;76:2559-2570.
[2] SHAMS AS, KYBA M. The satellite cell colony forming cell assay as a tool to measure self-renewal and differentiation potential. Methods Mol Biol. 2023;2640:45-55.
[3] 陈昱圻,郭昌龙,袁国红,等.衰老个体骨骼肌卫星细胞的研究进展[J].中国老年学杂志,2022,42(17):4354-4360.
[4] ZHU P, ZHANG C, GAO Y, et al. The transcription factor Slug represses p16INK4A and regulates murine muscle stem cell aging. Nat Commun. 2019;10(1):2568.
[5] HONG X, CAMPANARIO S, RAMÍREZ-PARDO I, et al. Stem cell aging in the skeletal muscle: the importance of communication. Ageing Res Rev. 2022;73:101528.
[6] MCKAY LK, WHITE JP. The AMPK/p27Kip1 pathway as a novel target to promote autophagy and resilience in aged cells. Cells. 2021;10(6):1430.
[7] KAUSHIK S, JUSTE YR, LINDENAU K, et al. Chaperone-mediated autophagy regulates adipocyte differentiation. Sci Adv. 2022;8(46):eabq2733.
[8] BRETT J O, ARJONA M, IKEDA M, et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat Metab. 2020;2(4):307-317.
[9] RUGOWSKA A, STAROSTA A, KONIECZNY P. Epigenetic modifications in muscle regeneration and progression of Duchenne muscular dystrophy. Clin Epigenetics. 2021;13(1):13.
[10] 李恭驰.IL-6通过Jak/Stat3通路促进萎缩肌肉的再生[D].武汉:华中科技大学, 2017.
[11] CAMPANARIO S, RAMÍREZ-PARDO I, HONG X, et al. Assessing autophagy in muscle stem cells. Front Cell Dev Biol. 2021;8:620409.
[12] AN Y, WANG G, DIAO Y, et al. A molecular switch regulating cell fate choice between muscle progenitor cells and brown adipocytes. Dev Cell. 2017;41(4):382-391.e5.
[13] DUMONT NA, WANG YX, RUDNICKI MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142(9):1572-1581.
[14] PRICE FD, VON MALTZAHN J, BENTZINGER CF, et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20(10):1174-1181.
[15] ONODERA Y, TERAMURA T, TAKEHARA T, et al. Transforming growth factor β-activated kinase 1 regulates mesenchymal stem cell proliferation through stabilization of Yap1/Taz proteins. Stem Cells. 2019;37(12):1595-1605.
[16] 刘晓光,陈佩杰,肖卫华.WNT信号通路在骨骼肌损伤修复过程中的作用及机制研究[J].生命的化学,2018,38(5):724-730.
[17] 陈茂,洪莉,李素廷,等.肌卫星细胞与其微环境作用及其活化增殖相关通路的研究进展[J].武汉大学学报(医学版),2019,40(4):683-688.
[18] 孔健达,穆玉晶,朱磊,等.骨骼肌再生过程中卫星细胞调控机制及其生态位信号的作用[J].中国组织工程研究,2024,28(7):1105-1111.
[19] GIULIANI G, ROSINA M, REGGIO A. Signaling pathways regulating the fate of fibro/adipogenic progenitors (FAPs) in skeletal muscle regeneration and disease. FEBS J. 2022;289(21):6484-6517.
[20] LUKJANENKO L, JUNG MJ, HEGDE N, et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat Med. 2016;22(8):897-905.
[21] MANOLE E, GAINA G, CEAFALAN LC, et al. Skeletal muscle stem cells in aging: asymmetric/symmetric division switching. Symmetry. 2022;14(12):2676.
[22] TOBIN SW, ALIBHAI FJ, WLODAREK L, et al. Delineating the relationship between immune system aging and myogenesis in muscle repair. Aging Cell. 2021;20(2):e13312.
[23] SCHÜLER SC, KIRKPATRICK JM, SCHMIDT M, et al. Extensive remodeling of the extracellular matrix during aging contributes to age-dependent impairments of muscle stem cell functionality. Cell Rep. 2021;35(10):109223.
[24] WANG Y, WELC SS, WEHLING-HENRICKS M, et al. Myeloid cell-derived tumor necrosis factor-alpha promotes sarcopenia and regulates muscle cell fusion with aging muscle fibers. Aging Cell. 2018;17(6):e12828.
[25] CSAPO R, GUMPENBERGER M, WESSNER B. Skeletal muscle extracellular matrix–what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol. 2020;11:253.
[26] MATHES S, FAHRNER A, GHOSHDASTIDER U, et al. FGF-2–dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A. 2021;118(37):e2021013118.
[27] MAHINDRAN E, WAN KAMARUL ZAMAN WS, AHMAD AMIN NOORDIN KB, et al. Mesenchymal stem cell-derived extracellular vesicles: hype or hope for skeletal muscle anti-frailty. Int J Mol Sci. 2023;24(9):7833.
[28] 龚丽景,潘秀清,付鹏宇.不同运动模式缓解衰老性骨骼肌萎缩的研究进展[J].山东体育学院学报,2023,39(2):84-91.
[29] GARCIA-PRAT L, PERDIGUERO E, ALONSO-MARTIN S, et al. FoxO maintains a genuine muscle stem-cell quiescent state until geriatric age. Nat Cell Biol. 2020;22(11): 1307-1318.
[30] FUKADA S, ITO N. Regulation of muscle hypertrophy: involvement of the Akt-independent pathway and satellite cells in muscle hypertrophy. Exp Cell Res. 2021; 409(2):112907.
[31] GATTAZZO F, LAURENT B, RELAIX F, et al. Distinct phases of postnatal skeletal muscle growth govern the progressive establishment of muscle stem cell quiescence. Stem Cell Reports. 2020;15(3):597-611.
[32] MUÑOZ‐CÁNOVES P, NEVES J, SOUSA‐VICTOR P. Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells. FEBS J. 2020;287(3):406-416.
[33] GUGLIUZZA MV, CRIST C. Muscle stem cell adaptations to cellular and environmental stress. Skeletal Muscle. 2022;12(1):1-12.
[34] GARCÍA-PRAT L, MARTÍNEZ-VICENTE M, PERDIGUERO E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37-42.
[35] IAKOVOU E, KOURTI M. A comprehensive overview of the complex role of oxidative stress in aging, the contributing environmental stressors and emerging antioxidant therapeutic interventions. Front Aging Neurosci. 2022;14:827900.
[36] ANTELO-IGLESIAS L, PICALLOS-RABINA P, ESTÉVEZ-SOUTO V, et al. The role of cellular senescence in tissue repair and regeneration. Mech Ageing Dev. 2021;198:111528.
[37] 郑莉芳,陈佩杰,周永战,等.老年骨骼肌再生能力受损的机制研究进展[J].生理科学进展,2017,48(5):393-397.
[38] KIM JW, KIM R, CHOI H, et al. Understanding of sarcopenia: from definition to therapeutic strategies. Arch Pharm Res. 2021;44(9-10):876-889.
[39] 王会玲,张丽萍,张金元.肌卫星细胞功能异常在慢性肾脏病骨骼肌消耗中的机制[J].肾脏病与透析肾移植杂志,2010,19(3):241-245,255.
[40] TIERNEY MT, STEC MJ, RULANDS S, et al. Muscle stem cells exhibit distinct clonal dynamics in response to tissue repair and homeostatic aging. Cell Stem Cell. 2018; 22(1):119-127.e3.
[41] HERNANDO-HERRAEZ I, EVANO B, STUBBS T, et al. Ageing affects DNA methylation drift and transcriptional cell-to-cell variability in mouse muscle stem cells. Nat Commun. 2019;10(1):4361.
[42] CARLSON ME, CONBOY IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6(3):371-382.
[43] LIAN D, CHEN MM, WU H, et al. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants. 2022;11(4):755.
[44] GILBERT PM, HAVENSTRITE KL, MAGNUSSON KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078-1081.
[45] SADTLER K, ESTRELLAS K, ALLEN BW, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016; 352(6283):366-370.
[46] BIFERALI B, PROIETTI D, MOZZETTA C, et al. Fibro-adipogenic progenitors cross-talk in skeletal muscle: the social network. Front Physiol. 2019;10:1074.
[47] CONTRERAS O, ROSSI FMV, THERET M. Origins, potency, and heterogeneity of skeletal muscle fibro-adipogenic progenitors-time for new definitions. Skelet Muscle. 2021;11(1):16.
[48] 周伸奥.Necroptosis在肌肉损伤修复过程中的功能研究[D].北京:中国科学院大学(中国科学院分子细胞科学卓越创新中心),2020.
[49] JUHAS M, ENGELMAYR GC JR, FONTANELLA AN, et al. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci U S A. 2014;111(15):5508-5513.
[50] 高广媛.雷帕霉素调控心肌梗死后心衰大鼠心肌细胞凋亡与自噬作用机制的研究[D].长春:吉林大学,2020.
[51] ZHU P, ZHANG C, GAO Y, et al. The transcription factor Slug represses p16INK4A and regulates murine muscle stem cell aging. Nat Commun. 2019;10(1):2568.
[52] COSGROVE BD, GILBERT PM, PORPIGLIA E, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20(3):255-264.
[53] WANG Y, WANG D, GUO Y, et al. The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen Ther. 2020;15:285-294.
[54] JUDSON RN, QUARTA M, OUDHOFF MJ, et al. Inhibition of methyltransferase Setd7 allows the in vitro expansion of myogenic stem cells with improved therapeutic potential. Cell Stem Cell. 2018;22(2):177-190.e7.
[55] OCAMPO A, REDDY P, MARTINEZ-REDONDO P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167(7):1719-1733.e12.
[56] EVANO B, GILL D, HERNANDO-HERRAEZ I, et al. Transcriptome and epigenome diversity and plasticity of muscle stem cells following transplantation. PLoS Genet. 2020;16(10):e1009022.
[57] QUARTA M, BRETT JO, DIMARCO R, et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol. 2016;34(7):752-759.
[58] 王晓玲,汪涛,汪雅妮,等.当归补血汤对移植肌卫星细胞γ射线照射小鼠早期造血重建作用的影响[J].时珍国医国药,2012,23(5):1059-1061.
[59] 王立妍,陈玉芳,王丹,等.当归补血汤促进异体移植的肌卫星细胞存活[J].中国组织化学与细胞化学杂志,2016,25(5):422-425.
[60] HEPPLE RT, RICE CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016, 594(8):1965-1978.
[61] DEL CARMEN ORTUÑO-COSTELA M, GARCÍA-LÓPEZ M, CERRADA V, et al. iPSCs: a powerful tool for skeletal muscle tissue engineering. J Cell Mol Med. 2019;23(6):3784-3794.
[62] TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872.
[63] JOANISSE S, NEDERVEEN JP, BAKER JM, et al. Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J. 2016;30(9):3256-3268.
[64] VINEL C, LUKJANENKO L, BATUT A, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24(9):1360-1371.
[65] MONDRAGON-GONZALEZ R, PERLINGEIRO RCR. Recapitulating muscle disease phenotypes with myotonic dystrophy 1 induced pluripotent stem cells: a tool for disease modeling and drug discovery. Dis Model Mech. 2018;11(7):dmm034728.
[66] VAN DER WAL E, HERRERO-HERNANDEZ P, WAN R, et al. Large-scale expansion of human iPSC-derived skeletal muscle cells for disease modeling and cell-based therapeutic strategies. Stem Cell Reports. 2018;10(6):1975-1990.
[67] KIM H, SELVARAJ S, KILEY J, et al. Genomic safe harbor expression of PAX7 for the generation of engraftable myogenic progenitors. Stem Cell Reports. 2021;16(1):10-19.
[68] MAFFIOLETTI SM, SARCAR S, HENDERSON ABH, et al. Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep. 2018;23(3):899-908.
[69] SELVARAJ S, MONDRAGON-GONZALEZ R, XU B, et al. Screening identifies small molecules that enhance the maturation of human pluripotent stem cell-derived myotubes. Elife. 2019;8:e47970.
[70] 王燕琳.基因修复DM1型患者ips细胞系及体外评估DM1患者骨骼肌功能指标的建立[D].郑州:郑州大学,2018.
[71] BORCHIN B, CHEN J, BARBERI T. Derivation and FACS-mediated purification of PAX3+/PAX7+ skeletal muscle precursors from human pluripotent stem cells. Stem Cell Reports. 2013;1(6):620-631.
[72] MAFFIOLETTI SM, GERLI MF, RAGAZZI M, et al. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat Protoc. 2015;10(7):941-958.
[73] DUMONT NA, BENTZINGER CF, SINCENNES MC, et al. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5(3):1027-1059.
[74] 谢娇娇,冯燕,姜丹阳,等.运动和衰老介导骨骼肌卫星细胞功能的研究进展[J].中国预防医学杂志,2020,21(10):1153-1161.
[75] NIU K, GUO H, GUO Y, et al. Royal jelly prevents the progression of sarcopenia in aged mice in vivo and in vitro. J Gerontol A Biol Sci Med Sci. 2013;68(12):1482-1492.
[76] OKUMURA N, TODA T, OZAWA Y, et al. Royal jelly delays motor functional impairment during aging in genetically heterogeneous male mice. Nutrients. 2018; 10(9):1191.
[77] ROMAGNOLI C, IANTOMASI T, BRANDI ML. Available in vitro models for human satellite cells from skeletal muscle. Int J Mol Sci. 2021;22(24):13221.
[78] 李江,包海姣,谢平波,等.衰老对骨骼肌卫星细胞增殖分化影响的研究进展[J].山东医药,2018,58(45):95-99. |