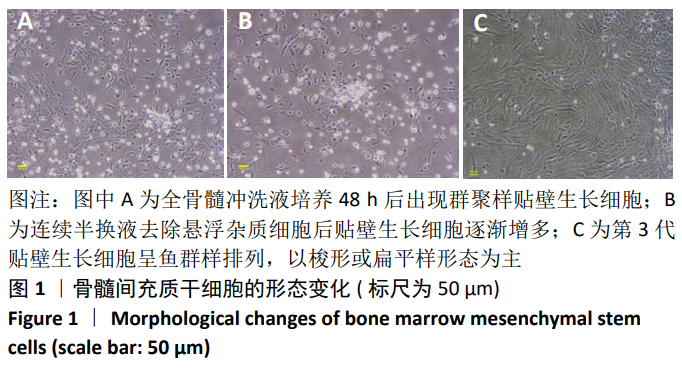
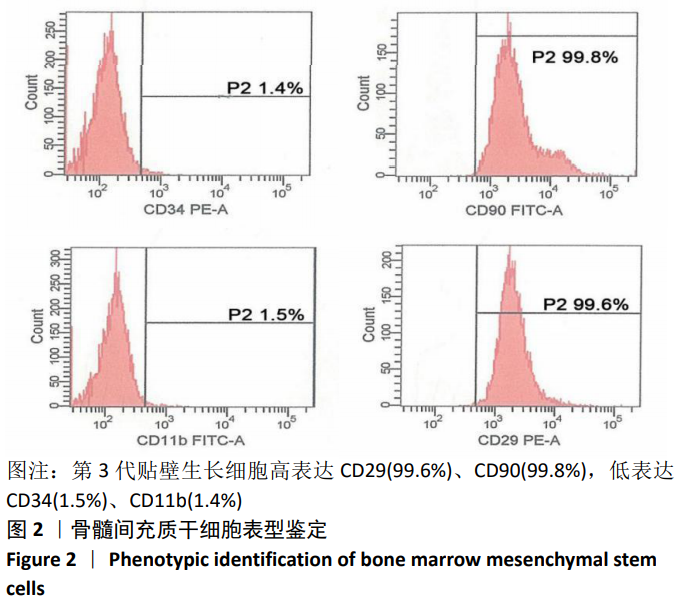
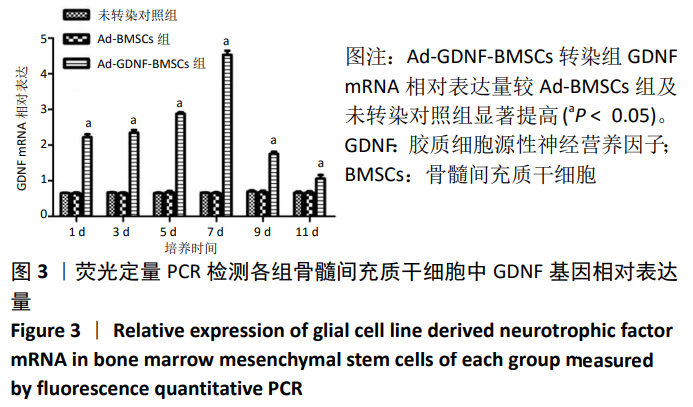
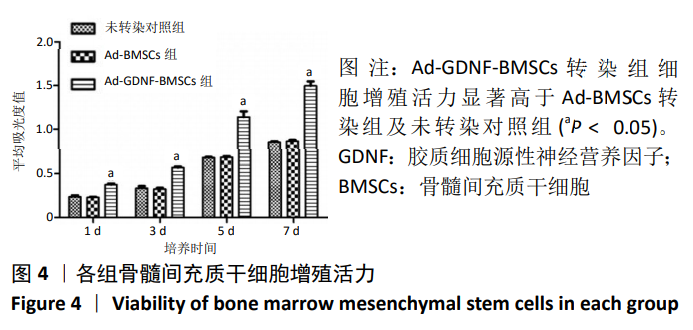
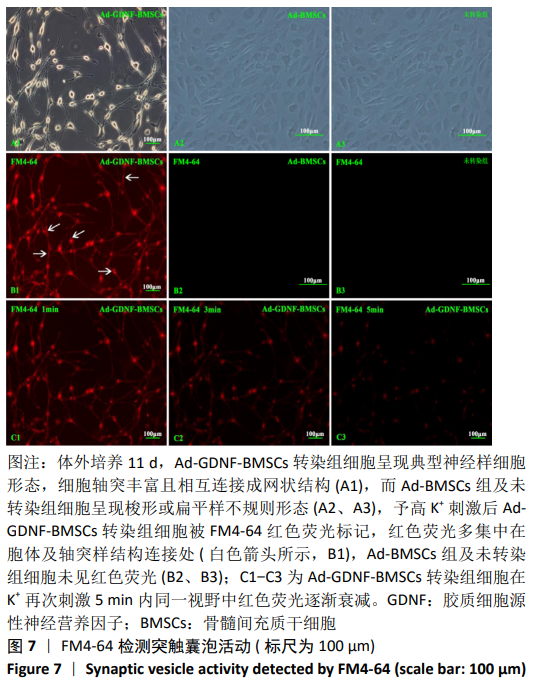
[1] SHAHREZAIE M, MANSOUR RN, NAZARI B, et al. Improved stem cell therapy of spinal cord injury using GDNF-overexpressed bone marrow stem cells in a rat model. Biologicals. 2017;50:73-80.
[2] LU Y, GAO H, ZHANG M, et al. Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury. Med Sci Monit. 2018; 23:1800-1811.
[3] YARAK S, OKAMOTO OK. Human adipose-derived stem cells: current challenges and clinical perspectives. An Bras Dermatol. 2010;85(5): 647-656.
[4] KAKABADZE Z, KIPSHIDZE N, MARDALEISHVILI K, et al. Phase 1 Trial of Autologous Bone Marrow Stem Cell Transplantation in Patients with Spinal Cord Injury. Stem Cells Int. 2016;2016:6768274.
[5] OH SK, CHOI KH, YOO JY, et al. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery. 2016;78(3):436-447.
[6] 黄成,刘元兵,杨建东,等.过表达胶质细胞神经营养因子基因转染骨髓间充质干细胞移植治疗脊髓损伤[J].中国组织工程研究, 2020,24(7):1037-1045.
[7] XIONG Y, ZHU JX, FANG ZY, et al. Coseeded Schwann Cells Myelinate Neurites From Differentiated Neural Stem Cells in neurotrophin-3-loaded PLGA Carriers. Int J Nanomedicine. 2012;7:1977-1989.
[8] TAKEDA A, SHAKUSHI Y, TAMANO H. Modification of hippocampal excitability in brain slices pretreated with a low nanomolar concentration of Zn2+. J Neurosci Res. 2015;93(11):1641-1647.
[9] FRIEDENSTEIN AJ, CHAILAKHJAN RK, LALYKINA KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393-403.
[10] FOUDAH D, MONFRINI M, DONZELLI E, et al. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. J Immunol Res. 2014;2014:987678.
[11] LOPEZ-VERRILLI MA, CAVIEDES A, CABRERA A, et al. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129-139.
[12] PU YJ, MENG K, GU CL, et al. Thrombospondin-1 modified bone marrow mesenchymal stem cells (BMSCs) promote neurite outgrowth and functional recovery in rats with spinal cord injury. Oncotarget. 2017; 8(56):96276-96289.
[13] ZENG X, QIU XC, MA YH, et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184-201.
[14] SI HP, LU ZH, LIN YL, et al.Transfect bone marrow stromal cells with pcDNA3.1-VEGF to construct tissue engineered bone in defect repair. Chin Med J (Engl). 2012;125(5):906-910.
[15] HU Y, ZHANG Y, TIAN K, et al. Effects of nerve growth factor and basic fibroblast growth factor dual gene modification on rat bone marrow mesenchymal stem cell differentiation into neuron-like cells in vitro. Mol Med Rep. 2016;13(1):49-58.
[16] NOORI-ZADEH A, MESBAH-NAMIN SA, TIRAIHI T, et al. Non-viral human proGDNF gene delivery to rat bone marrow stromal cells under ex vivo conditions. J Neurol Sci. 2014;339(1-2):81-86.
[17] DARABI S, TIRAIHI T, DELSHAD A, et al. In vitro non-viral murine pro-neurotrophin 3 gene transfer into rat bone marrow stromal cells. J Neurol Sci. 2017; 375:137-145.
[18] GONG Y, WANG H, XIA H. Stable transfection into rat bone marrow mesenchymal stem cells by lentivirus-mediated NT-3. Mol Med Rep. 2015; 11(1):367-373.
[19] LUO H, XU C, LIU Z, et al. Neural differentiation of bone marrow mesenchymal stem cells with human brain-derived neurotrophic factor gene-modified in functionalized self-assembling peptide hydrogel in vitro. J Cell Biochem. 2019; 120(3):2828-2835.
[20] TIAN W, HAN XG, LIU YJ, et al. Intrathecal epigallocatechin gallate treatment improves functional recovery after spinal cord injury by upregulating the expression of BDNF and GDNF.Neurochem Res. 2013; 38(4):772-779.
[21] SHEN Q, YIN Y, XIA QJ, et al. Bone Marrow stromal cells promote neuronal restoration in rats with traumatic brain injury: involvement of GDNF regulating BAD and BAX signaling. Cell Physiol Biochem. 2016; 38(2):748-762.
[22] GU WD, ZHANG FJ, XUE QS, et al. Bone mesenchymal stromal cells stimulate neurite outgrowth of spinal neurons by secreting neurotrophic factors. Neurol Res. 2012;34(2):172-180.
[23] JOSEPH M, DAS M, KANJI S, et al. Retention of stemness and vascul ogenic potential of human umbilical cord blood stem cells after repeated expansions on PES-nanofiber matrices. Biomaterials. 2014; 35(30):8566-8575.
[24] CADIGAN KM, LIU YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395-402.
[25] LIU F, KOHLMEIER S, WANG CY. Wnt signaling and skeletal development. Cell Signal. 2008;20(6):999-1009.
[26] VAN CAMP JK, BECKERS S, ZEGERS D, et al. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. 2014;10(2):207-229.
[27] FU HD, WANG HR, LI DH. BMP-7 accelerates the differentiation of rabbit mesenchymal stem cells into cartilage through the Wnt/β-catenin pathway. Exp Ther Med. 2017;14(6):5424-5428.
[28] KATO T, KHANH VC, SATO K, et al. Elevated expression of Dkk-1 by glucocorticoid treatment impairs bone regenerative capacity of adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2018;27(2): 85-99.
[29] GUO C, LIU R, ZHAO HB, et al. Wnt/β-catenin signal pathway mediated Salidroside induced directional differentiation from mouse mesenchymal stem cells to nerve cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(3):349-354.
[30] FENG N, HAN Q, LI J, et al. Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation. Stem Cells Dev. 2014;23(5):515-529.
[31] 孙经淞,周雪颖,曲淑贤,等.骨髓间充质干细胞移植对脊髓损伤的修复作用[J].中国组织工程研究, 2018,22(1):59-64.
[32] YUE W, YAN F, ZHANG YL, et al. Differentiation of rat bone marrow mesenchymal stem cells into neuron-like cells in vitro and co-cultured with biological scaffold as transplantation carrier. Med Sci Monit. 2016; 22:1766-1772.
[33] LIN WW, LI M, LI Y, et al. Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci. 2014;35(3):449-457.
[34] LI D, HÉRAULT K, OHEIM M, et al. FM dyes enter via a store-operated calcium channel and modify calcium signaling of cultured astrocytes. Proc Natl Acad Sci U S A. 2009;106(51):21960-21965.
[35] NIEWEG K, ANDREYEVA A, VAN STEGEN B, et al. Alzheimer’s disease-related amyloid-β induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 2015;6(4):e1709.
[36] LIU C, HU Q, JING J, et al. Regulator of G protein signaling 5 (RGS5) inhibits sonic hedgehog function in mouse cortical neurons. Mol Cell Neurosci. 2017;83:65-73.
[37] ANKOLEKAR SM, SIKDAR SK. Early postnatal exposure to lithium in vitro induces changes in AMPAR mEPSCs and vesicular recycling at hippocampal glutamatergic synapses. J Biosci. 2015;40(2):339-354.
[38] DARABI S, TIRAIHI T, DELSHAD A, et al. Creatine enhances transdifferentiation of bone marrow stromal cell-derived neural stem cell into GABAergic neuron-Like cells characterized with differential gene expression. Mol Neurobiol. 2017;54(3):1978-1991.
[39] DARVISHI M, TIRAIHI T, MESBAH-NAMIN SA, et al. Motor neuron transdifferentiation of neural stem cell from adipose-derived stem cell characterized by differential gene expression.Cell Mol Neurobiol. 2017;37(2):275-289.
[40] LIU W, WANG YX, GONG FY, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36(3):469-484.
[41] CHEDLY J, SOARES S, MONTEMBAULT A, et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials. 2017;138:91-107.
|