| [1] Giannoudis PV, Gudipati S, Harwood P, et al. Long bone non-unions treated with the diamond concept: a case series of 64 patients. Injury. 2015;46 Suppl 8:S48-54. [2] Dumic-Cule I, Pecina M, Jelic M, et al. Biological aspects of segmental bone defects management. Int Orthop. 2015; 39(5):1005-1011. [3] Carreira AC, Zambuzzi WF, Rossi MC, et al. Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine. Vitam Horm. 2015;99:293-322. [4] Curtin CM, Tierney EG, McSorley K, et al. Combinatorial gene therapy accelerates bone regeneration: non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv Healthc Mater. 2015;4(2):223-227. [5] Liu F, Ferreira E, Porter RM, et al. Rapid and reliable healing of critical size bone defects with genetically modified sheep muscle. Eur Cell Mater. 2015;30:118-130. [6] Helary C, Desimone MF. Recent advances in biomaterials for tissue engineering and controlled drug delivery. Curr Pharm Biotechnol. 2015;16(7):635-645. [7] Beck-Broichsitter BE, Werk AN, Smeets R, et al. Targeting gene expression during the early bone healing period in the mandible: A base for bone tissue engineering. J Craniomaxillofac Surg. 2015;43(8):1452-1460. [8] Guan J, Zhang J, Li H, et al. Human Urine Derived Stem Cells in Combination with β-TCP Can Be Applied for Bone Regeneration. PLoS One. 2015;10(5):e0125253. [9] Wan W, Zhang S, Ge L, et al. Layer-by-layer paper-stacking nanofibrous membranes to deliver adipose-derived stem cells for bone regeneration. Int J Nanomedicine. 2015;10: 1273-1290. [10] Bolander J, Ji W, Geris L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur Cell Mater. 2016;31:11-25. [11] Bolander J, Ji W, Leijten J, et al. Healing of a Large Long-Bone Defect through Serum-Free In Vitro Priming of Human Periosteum-Derived Cells. Stem Cell Reports. 2017; 8(3):758-772. [12] Li CJ, Madhu V, Balian G, et al. Cross-Talk Between VEGF and BMP-6 Pathways Accelerates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J Cell Physiol. 2015; 230(11):2671-2682. [13] Wang L, Zhang YG, Wang XM, et al. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem Biol Interact. 2015;242:255-261. [14] Abagnale G, Steger M, Nguyen VH, et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials. 2015;61: 316-326. [15] Di Benedetto A, Brunetti G, Posa F, et al. Osteogenic differentiation of mesenchymal stem cells from dental bud: Role of integrins and cadherins. Stem Cell Res. 2015;15(3): 618-628. [16] Wöltje M, Böbel M, Heiland M, et al. Purmorphamine and oxysterols accelerate and promote osteogenic differentiation of mesenchymal stem cells in vitro. In Vivo. 2015;29(2): 247-254. [17] Chung JE, Park JH, Yun JW, et al. Cultured Human Periosteum-Derived Cells Can Differentiate into Osteoblasts in a Perioxisome Proliferator-Activated Receptor Gamma-Mediated Fashion via Bone Morphogenetic Protein signaling. Int J Med Sci. 2016;13(11):806-818. [18] Kim YK, Nakata H, Yamamoto M, et al. Osteogenic Potential of Mouse Periosteum-Derived Cells Sorted for CD90 In Vitro and In Vivo. Stem Cells Transl Med. 2016;5(2):227-234. [19] Sung IY, Park BC, Hah YS, et al. FOXO1 Is Involved in the Effects of Cigarette Smoke Extract on Osteoblastic Differentiation of Cultured Human Periosteum-derived Cells. Int J Med Sci. 2015;12(11):881-890. [20] Bobinac D, Spanjol J, Marinovi? M, et al. Expression of bone morphogenetic proteins, cartilage-derived morphogenetic proteins and related receptors in normal and osteoarthritic human articular cartilage. Coll Antropol. 2008;32 Suppl 2: 83-87. [21] Jin J, Wang J, Huang J, et al. Transplantation of human placenta-derived mesenchymal stem cells in a silk fibroin/hydroxyapatite scaffold improves bone repair in rabbits. J Biosci Bioeng. 2014;118(5):593-598. [22] Sinlapabodin S, Amornsudthiwat P, Damrongsakkul S, et al. An axial distribution of seeding, proliferation, and osteogenic differentiation of MC3T3-E1 cells and rat bone marrow-derived mesenchymal stem cells across a 3D Thai silk fibroin/gelatin/hydroxyapatite scaffold in a perfusion bioreactor. Mater Sci Eng C Mater Biol Appl. 2016;58: 960-970. [23] Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18(2):213-225. [24] Dos Santos Pereira R, Boos FB, Gorla LF, et al. Maxillary Sinus Elevation Surgery with ChronOS and Autogenous Bone Graft: Analysis of Histometric and Volumetric Changes. Int J Periodontics Restorative Dent. 2016;36(6):885-892. [25] Lareau CR, Deren ME, Fantry A, et al. Does autogenous bone graft work? A logistic regression analysis of data from 159 papers in the foot and ankle literature. Foot Ankle Surg. 2015;21(3):150-159. [26] Nauth A, Lane J, Watson JT, et al. Bone Graft Substitution and Augmentation. J Orthop Trauma. 2015;29 Suppl 12: S34-38. [27] Durst A, Clibbon J, Davis B. Distal tibial fractures are a poorly recognised complication with fibula free flaps. Ann R Coll Surg Engl. 2015;97(6):409-413. [28] Lonie S, Herle P, Paddle A, et al. Mandibular reconstruction: meta-analysis of iliac- versus fibula-free flaps. ANZ J Surg. 2016;86(5):337-342. [29] Van Genechten ML, Batstone MD. The relative survival of composite free flaps in head and neck reconstruction. Int J Oral Maxillofac Surg. 2016;45(2):163-166. [30] Micev AJ, Kalainov DM, Soneru AP. Masquelet technique for treatment of segmental bone loss in the upper extremity. J Hand Surg Am. 2015;40(3):593-598. [31] Morelli I, Drago L, George DA, et al. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47 Suppl 6:S68-S76. [32] Tarchala M, Harvey EJ, Barralet J. Biomaterial-Stabilized Soft Tissue Healing for Healing of Critical-Sized Bone Defects: the Masquelet Technique. Adv Healthc Mater. 2016;5(6):630-640. [33] Guo ZM, ShangGuan TC, Zhang M, et al. Prevention and treatment of the related complications of tibial fractures bone defect by bone transport. Zhongguo Gu Shang. 2016;29(8): 756-760. [34] Tsitskaris K, Havard H, Bijlsma P, et al. Internal bone transport using a cannulated screw as a mounting device in the treatment of a post-infective ulnar defect. Strategies Trauma Limb Reconstr. 2016;11(1):63-67. [35] Chen L, Li B, Xiao X, et al. Preparation and evaluation of an Arg-Gly-Asp-modified chitosan/hydroxyapatite scaffold for application in bone tissue engineering. Mol Med Rep. 2015; 12(5):7263-7270. [36] Nguyen DT, Burg KJ. Bone tissue engineering and regenerative medicine: targeting pathological fractures. J Biomed Mater Res A. 2015;103(1):420-429. [37] De Francesco F, Ricci G, D'Andrea F, et al. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev. 2015;21(6):572-584. [38] Romagnoli C, Zonefrati R, Galli G, et al. In Vitro Behavior of Human Adipose Tissue-Derived Stem Cells on Poly(ε-caprolactone) Film for Bone Tissue Engineering Applications. Biomed Res Int. 2015;2015:323571. [39] Zanetti AS, McCandless GT, Chan JY, et al. Characterization of novel akermanite: poly-?-caprolactone scaffolds for human adipose-derived stem cells bone tissue engineering. J Tissue Eng Regen Med. 2015;9(4):389-404. [40] Yin X, Xu H, Jiang Y, et al. The effect of lentivirus-mediated PSPN genetic engineering bone marrow mesenchymal stem cells on Parkinson's disease rat model. PLoS One. 2014;9(8): e105118. [41] Liu X, Joshi S, Ravishankar B, et al. Bone morphogenetic protein signaling in rotator cuff muscle atrophy and fatty infiltration. Muscles Ligaments Tendons J. 2015;5(2):113-119. [42] Yin Y, Wang Y. Association of BMP-14 rs143383 ploymorphism with its susceptibility to osteoarthritis: A meta-analysis and systematic review according to PRISMA guideline. Medicine (Baltimore). 2017;96(42):e7447. |
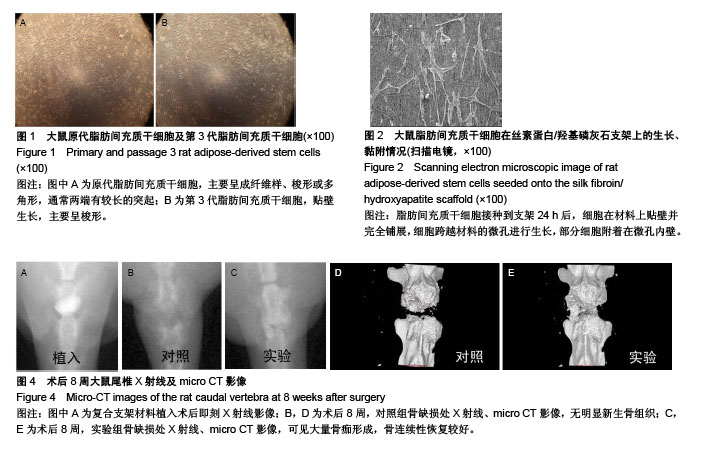
.jpg)
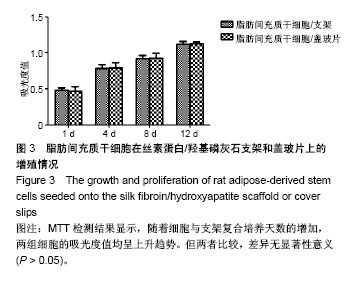
.jpg)