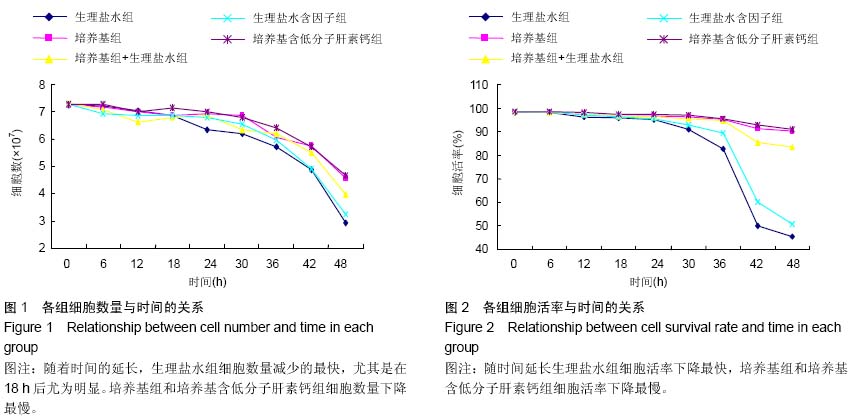
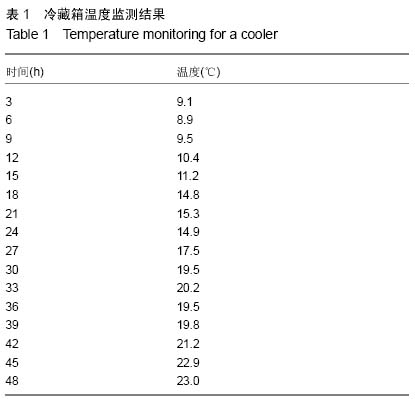
| [1] Gamie Z, MacFarlane RJ, Tomkinson A,et al. Skeletal tissue engineering using mesenchymal or embryonic stem cells: clinical and experimental data. Expert Opin Biol Ther. 2014; 14(11):1611-1639.
[2] Wang G, Li Y, Wang Y, et al. Roles of the co-culture of human umbilical cord Wharton's jelly-derived mesenchymal stem cells with rat pancreatic cells in the treatment of rats with diabetes mellitus. Exp Ther Med. 2014;8(5):1389-1396.
[3] Woodworth TG, Furst DE. Safety and feasibility of umbilical cord mesenchymal stem cells in treatment-refractory systemic lupus erythematosus nephritis: time for a double-blind placebo-controlled trial to determine efficacy. Arthritis Res Ther. 2014;16(4):113.
[4] Wen Y, Gu W, Cui J,et al. Platelet-rich plasma enhanced umbilical cord mesenchymal stem cells-based bone tissue regeneration. Arch Oral Biol. 2014;59(11):1146-1154.
[5] Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2014;200(1):31-41.
[6] Cui B, Li E, Yang B, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7(5):1233-1236.
[7] Xiong N, Zhang Z, Huang J,et al. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 2011;18(4): 394-402.
[8] Wang X, Hu H, Hua R,et al. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: pilot study on the correlation of efficacy and hereditary factors. Cytotherapy. 2015;17(2): 224-231.
[9] Lee HJ, Lee JK, Lee H, et al. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci Lett. 2010;481(1):30-35.
[10] El-Jawhari JJ, El-Sherbiny YM, Jones EA,et al. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM. 2014; 107(7):505-514.
[11] Xue HL, Zeng WZ, Wu XL, et al. Clinical therapeutic effects of human umbilical cord-derived mesenchymal stem cells transplantation in the treatment of end-stage liver disease. Transplant Proc. 2015;47(2):412-418.
[12] Santos Nascimento D, Mosqueira D, Sousa LM,et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther. 2014;5(1):5.
[13] Chao YH, Wu HP, Wu KH, et al. An increase in CD3+CD4+CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS One. 2014;9(10):e110338.
[14] Wu KH, Tsai C, Wu HP, et al. Human application of ex vivo expanded umbilical cord-derived mesenchymal stem cells: enhance hematopoiesis after cord blood transplantation. Cell Transplant. 2013;22(11):2041-2051.
[15] Auletta JJ, Eid SK, Wuttisarnwattana P,et al. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells. 2015;33(2):601-614.
[16] 《中国组织工程研究与临床康复》杂志社学术部. 《干细胞临床转化指南》:干细胞临床应用标准与规则[J].中国组织工程研究与临床康复,2010,14(36):6798-6799.
[17] 国家食品药品监督管理局.《人体细胞治疗研究和制剂质量控制技术指导原则》[EB/OL]. http://www.sda.gov.cn/ WS01/ CL0237/15709.html, 2003-03-20
[18] Bosse R, Singhofer-Wowra M, Rosenthal F, et al. Good manufacturing practice production of human stem cells for somatic cell and gene therapy. Stem Cells. 1997;15 Suppl 1: 275-280.
[19] 毕薇薇,郭立斌,李志刚.人脐带源间充质干细胞的培养及生物学性状[J].中国现代医学杂志,2009,19(10):1511-1513.
[20] Eftekhar-Vaghefi SH, Zahmatkesh L, Salehinejad P, et al. Evaluation of neurogenic potential of human umbilical cord mesenchymal cells; a time- and concentration-dependent manner. Iran Biomed J. 2015;19(2):82-90.
[21] Frausin S, Viventi S, Verga Falzacappa L, et al. Wharton's jelly derived mesenchymal stromal cells: Biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta Histochem. 2015 Mar 3. [Epub ahead of print]
[22] Coskun H, Can A. The assessment of the in vivo to in vitro cellular transition of human umbilical cord multipotent stromal cells. Placenta. 2015;36(2):232-239.
[23] Maleki M, Ghanbarvand F, Reza Behvarz M, et al. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118-126.
[24] Shaer A, Azarpira N, Aghdaie MH, et al. Isolation and characterization of Human Mesenchymal Stromal Cells Derived from Placental Decidua Basalis; Umbilical cord Wharton's Jelly and Amniotic Membrane. Pak J Med Sci. 2014;30(5):1022-1026.
[25] Mueller AA, Forraz N, Gueven S, et al. Osteoblastic differentiation of Wharton jelly biopsy specimens and their mesenchymal stromal cells after serum-free culture. Plast Reconstr Surg. 2014;134(1):59e-69e.
[26] Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195-202.
[27] 张可华,纳涛,刘静,等.建立间充质干细胞制剂质量控制评价体系[C].南宁:2013年中国药学大会暨第十三届中国药师周论文集, 2013-11-02.
[28] 孙瑞婷,陈瑶瑶,王华,等. 脐带来源间充质干细胞的制备及其质量检定[J].中国医药生物技术,2013,8(6):401-407.
|