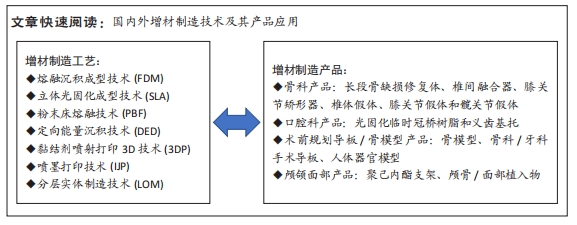
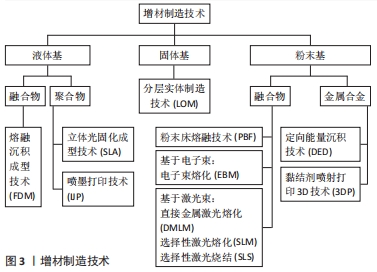
根据OLIVEIRA等[11-12]的说法,增材制造技术的分类与三维打印设备完全相关。基于所用材料状态的增材制造技术分类如图3所示,不同增材制造技术的技术特征见表1,该文针对各增材技术的具体情形概括如下:
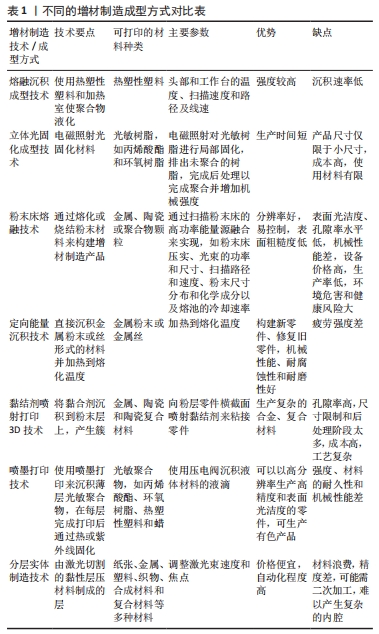
2.1 熔融沉积成型技术 熔融沉积成型技术又称为熔丝成型或熔丝制造,该过程使用热塑性塑料和加热室使聚合物液化,材料沉积通过挤出机头进行,挤出机头通过添加材料细丝沿x轴和y轴移动;完成一层后,沉积平台沿z方向向下移动以构建下一层,依此类推,直到零件完成[13],主要变量是头部和工作台的温度、扫描速度和路径及线速。如今熔融沉积成型的许多零件比通过相同材料传统工艺制造的零件表现出更高的强度。将熔融沉积成型热塑性材料的屈服强度值与模制材料的屈服强度值进行比较,显示熔融沉积成型的强度值保持在22-71 MPa之间,而注塑模具的强度值在20-60 MPa之间[14]。
熔融沉积成型技术的主要限制之一是分辨率取决于电线厚度,目前最小为0.127 mm[6];另一个限制是沉积速率,由于打印台的惯性,与其他增材制造技术相比该技术沉积速率非常低。此外,该工艺主要用于聚合物,其中最常见的是热塑性塑料,主要应用是生产具有生物惰性抗性聚合物(如聚醚醚酮)的支架[15],该聚合物可与羟基磷灰石结合使用以诱导骨修复中的细胞附着。该技术在面部重建和全关节置换术中的关节垫已经有商业应用[16],但用于膝关节和髋关节等负荷更大关节的全聚醚醚酮植入物尚未准备好用于商业应用[17]。
生物降解和多孔支架也可以通过使用聚乳酸和聚己内酯挤出制造,调整工艺参数有助于最大限度地改善它们的机械性能较低这一缺点。DE CIURANA等[18]研究表明可以选择不同的熔融沉积路径来定制孔隙率,并在相互连接的孔隙中实现强度和细胞生长之间的良好组合[19],这种平衡可以通过特殊的几何形状和拓扑优化进一步改善[20-21]。WARIS等[22]生产了多孔可生物降解的支架以促进迷你猪的纤维组织生长,这可能对小的人类关节有用,例如手指中的关节。DUAN等[23]进一步用干细胞填充毛孔获得了兔骨软骨修复更快的方法。聚己内酯线材的较低熔化温度也推动使用熔融沉积成型技术用于打印活细胞,如ZHENG等[24]在富含干细胞和结缔组织生长因子的水凝胶基质中打印山羊半月板。
2.2 立体光固化成型技术 立体光固化成型由于电磁照射光固化材料而固化,是最广泛使用的增材制造技术之一,也被称为液体树脂的光聚合,最初由3D Systems公司销售。该工艺的原理是通过沿X轴和Y轴移动的紫外激光束对光敏树脂进行局部固化,也可以使用其他促进聚合的光源,例如电子束、辐射、高能粒子束、X射线、UV光束和常规UV光[25],光束聚焦在树脂浸入容器上,以构建先前计算层的轮廓;每层完成后,材料支撑平台沿Z轴向下移动,开始建造新层,重复此过程,直到零件完成;之后,平台升高,排出未聚合的树脂[26],制造部件的聚合度在80%-90%之间[25],这一步意味着需完成后处理以完成聚合并增加机械强度。在这个阶段,成品零件保存在保持紫外线发射的烤箱中。此外,去除支撑材料也是后处理的一部分[27]。
立体光固化成型工艺通常使用的材料基于丙烯酸酯和环氧树脂,它们为制造功能部件提供了合适的机械强度。此外,机械强度可以量化并表示为28-78 MPa的屈服强度[28]。关于该技术的精度,目前发现层分辨率高达50 μm,而扫描精度通常约为25 μm,在这种情况下物体的加工速度可以达到35 m/s。然而也有特定情况,例如微甾体光刻,尽管打印速度会显著降低,但其层分辨率(z)和扫描精度(x-y)的值可以分别达到0.1 μm和0.25 μm[29]。
立体光固化成型工艺的主要优点是生产时间比熔融沉积成型技术更短,这是因为激光扫描速度更高,缺点包括产品的尺寸仅限于小尺寸(大约2英尺立方体的大小),另一个缺点是成本,因为光敏聚合物的成本在300-500美元之间,此外还有设备本身的价值。与熔融沉积成型技术相比,立体光固化成型中使用的材料甚至更加有限,因为并非所有热塑性塑料都可以从光固化树脂中轻松加工[6]。
立体光固化成型在生物医学中用于生产支架,许多产品将分散在树脂中的磷酸钙结合制成用于骨修复的多孔陶瓷[30]。ZHOU等[31]用粉末状β-磷酸三钙、树脂和分散剂的混合物生产了脊柱形的基体,烧结后孔隙率约为14%、平均晶粒尺寸为0.7 μm,达到骨支架的适当结构。与更好的立体光固化成型分辨率相结合,这些特性优于用于相同应用程序的其他增材制造过程,例如选择性激光熔化;然而,选择性激光熔化可以更快地达到相同的结构,并且无需烧结,这意味着产品可以更便宜。用于手术计划和教学目的的纯聚合物支架和模型也是该技术的可能用途,但功能多和价格实惠的熔融沉积成型技术将更具优势。
2.3 粉末床熔融技术 粉末床熔融技术通过熔化或烧结粉末材料来构建增材制造产品,这些层的构建是通过金属、陶瓷或聚合物颗粒沿x-y轴扫描粉末床的高功率能量源融合来实现的。每层完成后,施工平台沿z轴移动,材料铺入床中形成新层[32]。
能量源的两种类型分别是激光束和电子束,它们具有不同的工艺要求,并且对于相似的参数产生不同的零件特性,激光束熔化包括直接金属激光熔化、选择性激光熔化和选择性激光烧结。激光熔化和电子束熔化都可以通过在层间扫描来预热粉末床,以尽量减少热变形并促进与前一层的融合[6]。
基于激光设备可以实现更好的分辨率,因为光斑可以聚焦到更小的直径,这允许生产具有严格尺寸公差的极薄壁。另一方面,电子束熔化依赖于由30-60 kV电压加速的电子[33],与激光相比,它通常具有更高的功率输出和功率转换效率、可产生更大的熔池,使其能够比前一种工艺更快地生产大批量零件[34]。这个过程需要一个昂贵的高真空室来避免电子的分散[32],但是它允许加工高反应性材料,就像预合金粉末金属一样[35]。电子控制螺线管实现的约1 000 m/s的扫描速度远大于由移动镜机械控制的10 m/s激光,后者发生在惰性气体气氛下,以避免熔池和粉末氧化,有助于冷却腔室[36],这代表了对激光熔池的更好控制,有助于降低表面粗糙度[37]。
这2个过程都取决于许多变量,例如粉末床压实、光束的功率和尺寸[38]、扫描路径和速度[39]、粉末尺寸分布和化学成分以及熔池的冷却速率,由于参数众多,零件的最终特性仍存在很大差异。该技术可实现的表面光洁度不如立体光固化成型技术[40],孔隙率水平(根据工艺参数和颗粒材料特性)可能在最终物体体积的50%-90%之间变化,电子束熔化的结果更好。因此,机械性能变化明显,屈服强度在5.5-90 MPa之间[14],这可以通过使用热等静压来纠正,但这个过程也非常昂贵。
这些工艺的优点是:增材制造工艺中金属产品的最佳分辨率和公差;可以加工的材料种类繁多,主要包括金属和陶瓷,甚至可以混合不同的粉末。然而,使用电子束熔化需要电源床的导电性[41]。目前一个重要的问题是,大部分打印设备制造商同时提供配合使用的原材料,原材料缺乏成熟的市场,阻碍了买方的自主权。
电子束熔化的一些主要缺点是设备价格高和低生产率[42],粉末基技术的一个重要缺点是环境和健康风险[43],高表面体积比使其极易燃,需要特殊的程序来储存和运输材料[44]。此外,细小的金属颗粒容易在空气中传播,暴露的操作人员可能会出现呼吸道炎症[45]。粉末的高成本也是另一个问题,因为它们是通过能量密集型方法制造的,例如气体或等离子体雾化[46]。
选择性激光熔化和电子束熔化已被广泛用于制造硬组织替代的金属和金属陶瓷植入物,DALLAGO等[47]研究表明,调整工艺参数以控制尺寸误差是该过程的主要目标之一。许多商业植入物已经由选择性激光熔化、选择性激光烧结和电子束熔化制造,例如用于髋关节置换术的髋臼组件、口腔种植用基台和膝关节植入物。
2.4 定向能量沉积技术 直接沉积粉末或金属丝形式的材料并加热到熔化温度,是定向能量沉积工艺的工作原理。热源包括激光和等离子弧,其方式与焊接操作中使用的方式相同。然而,即使是更薄的线材原料也会产生比电子束熔化和选择性激光熔化大得多的熔池。具有更好分辨率和速度的变化是带有粉末的激光近净成型技术[6],其中基板在激光束下移动,沉积薄的横截面材料以创建所需的几何形状,按顺序连续层沉积以构建增材制造产品[3]。它的优点是除了构建新零件还可修复旧零件,并且很容易实现良好的机械性能[6]。然而,激光近净成型技术需要一些后精加工工艺来确保更好的质量,例如铣削、车削和抛光。
这种增材制造工艺在医疗领域的主要应用是生产用于整形外科手术的钛植入物,是粉末床熔融工艺的替代品。ATTAR等[48]比较了激光近净成型技术和选择性激光熔化生产产品的机械性能,发现后者具有更好的抗拉性。这是由于选择性激光熔化中更快的冷却速率导致钛的微观结构更精细,这也对耐腐蚀性和耐磨性更好;与通过铸造或热压等传统方法制造的钛产品相比,这些特性仍然被发现更好[49]。该工艺的一个主要问题是HARUN等[50]所指出的疲劳强度,这是由于孔隙之间形成微裂纹。定向能量沉积工艺落后于粉末床熔融制造,例如选择性激光熔化或电子束熔化可实现多功能性和更好的公差与粗糙度。
2.5 黏结剂喷射打印3D技术 黏结剂喷射打印3D技术由麻省理工学院授权,商业名称为Prometal,并且基于将黏结剂沉积到粉末层上产生簇,在这个过程中含尘储液罐提升平台,而滚筒分布在工件施工平台上;对于层的生成,喷墨头沿x-y移动,将黏性材料打印到粉尘层上。因为它与用于二维纸张打印的喷墨打印过程相似,因此该过程称为黏结剂喷射打印3D技术[51]。根据NEMATOLLAHI等[6]的说法,材料首先通过喷洒水滴来稳定,以避免在被黏合剂撞击时过度干扰。此外,在沉积过程中可以进行渗透,因为黏结剂可能不足以保证足够的生坯阻力。在依次施加层后,去除未结合的粉尘,并对所谓的“生坯”部分进行排胶和烧结。起初,温度保持在较低的值,仅足以蒸发黏合剂,而后来使用高温来促进扩散并进一步加强材料的黏合。在某些情况下,在后处理时进行热等静压以减少孔隙率,这可能进一步导致屈服强度高于400 MPa。
黏结剂喷射打印3D工艺可应用于生产金属、陶瓷和陶瓷复合材料,优点是快速实现和制造材料成本低。关于沉积厚度,该技术提供的层厚度在0.089-0.2 mm之间,而分辨率在600×540 每英寸点数(DPI)之间,这种整体精度约为0.125 mm[6]。就像在电子束熔化和选择性激光熔化中一样,该工艺可以与同一零件中的不同粉末一起使用,以在整个体积中生产不同性能的合金和复合材料[40]。
在打印时间方面,黏结剂喷射打印3D技术是最快的技术之一,但也存在一些限制,例如烧结后的孔隙率高、尺寸限制和后处理阶段太多。虽然整合的喷墨技术降低了打印机的成本,但大多数烧结炉和热等静压设备都相当昂贵[6]。就像选择性激光烧结和电子束熔化一样,粉末需要小心处理,与黏合剂和烧结相关的许多变量进一步增加了调整材料和工艺参数时的复杂性[51]。
通过黏结剂喷射进行3D打印的产品主要应用于生产用于骨和关节手术的金属和陶瓷部件或支架[52],常见的使用材料是Ti-6Al-4V合金和磷酸钙,但也有可生物降解的铁合金相关研究[53]。该技术也应用于构建用于手术计划和医学教学目的的3D模型[54]。
2.6 喷墨打印技术 喷墨打印技术包括使用喷墨打印来沉积薄层光敏聚合物(高达16 μm),在每层完成打印后通过热或紫外线固化[6]。喷墨头在“x”和“y”上移动,每层完成后施工平台沿z方向移动[55]。喷墨技术被广泛开发用于传统的纸张2D打印,使用压电阀沉积液体材料的液滴,并且该增材制造技术采用了相同的原理[56-57]。
喷墨打印技术通常使用的材料是丙烯酸酯、环氧树脂、热塑性塑料和蜡,这些材料在凝固过程方面彼此不同,这使得这些技术可以分为两组:可光聚合的喷墨打印和可热聚合的喷墨打印。例如,由Objet开发的EDEN设备是一种喷墨打印类型的光固化材料,其喷墨头通过1 536个单独的喷嘴沉积材料,这些喷嘴同时排列在15 μm层中。在这个过程中,最终部分的聚合程度通常高于立体光固化成型,因此没有后处理的需要[58]。
喷墨打印工艺的主要长处是可以以高分辨率生产高精度和表面光洁度的零件,该技术得到了很好的整合、降低了设备和原料的价格。此外,该技术一项强大的功能是改变层内的墨水源,以在聚合物支架中生产有色部件、聚合物复合材料和种子活细胞[59],缺点是强度、材料的耐久性和机械性能差,此外还有立体光固化成型在光固化聚合物方面的相同限制。与立体光固化成型和熔融沉积成型技术相比,基于该技术构建的产品被认为更脆弱。
喷墨打印在生物医学中的应用与一些黏结剂喷射非常相似,普遍趋势是出于教学目的和手术计划而生产模型[60],并且由于比立体光固化成型更好的几何公差,可用于牙托的快速生产[61]。
2.7 分层实体制造技术 分层实体制造技术结合了增材和减材技术,用于构建由激光切割的黏性层压材料制成的层[3,12]。调整激光束速度和焦点使切割深度与层的厚度完全对应,从而避免损坏底层材料。
分层实体制造技术可以使用纸张、金属、塑料、织物、合成材料和复合材料等多种材料。分层实体制造技术价格便宜,可以自动化,几乎不需要操作员的关注,因此可以轻松生产大型零件。但是,它存在精度问题,导致了尺寸稳定性问题,它可能会产生一些影响产品质量的内部空腔;除此之外,还需要后期生产时间来消除浪费,在某些情况下需要二次加工才能更准确地生成零件[62]。该技术的缺点是材料浪费(由加法和减法技术相结合造成)及难以产生复杂的内腔[6]。此外在使用金属的情况下,结合的电阻远低于合金本身,因此与其他增材制造工艺相比吸引力较低。
关于分层实体制造技术在生物医学中的应用,分层实体制造技术的应用主要局限于制造教学和手术计划的模型[63],未来可能开发一种应用陶瓷材料制造的微孔支架。ZHANG等[64]能够生产具有80 μm规则孔和50%整体孔隙率的氧化铝支架。
2.8 增材制造产品在临床中的应用 全球增材制造相关的新工艺、新原理、新材料、新应用不断涌现,空间3D打印[4,65]、电子3D打印、细胞3D打印[5]、微纳3D打印[6]、4D打印等新概念层出不穷[6]。增材制造允许工程师打印各种假体医疗设备[66],甚至人体器官,如肾脏、耳朵和手指骨[67]。增材制造的康复器具、手术导航以及医疗植入物等为代表,极具应用前景。
增材制造在医学上的主流应用可分为以下5种类型[68]:①物理器官模型、手术导板和体外医疗器械:其组成材料的生物相容性要求通常取决于与人体的相互作用程度。器官模型可能不具有生物相容性,但手术导板(如切割/钻孔导板)和一些器械(如正畸和假肢)仍需要生物相容性测试。②植入物:组成材料应是生物相容的。③组织支架:必须具有生物相容性、可降解性和可吸收性。④体外组织模型:具有生物活性,通常由含有细胞的生物材料构建。⑤工程生命系统。
该文主要综述性说明增材制造技术在临床应用领域的研究进展,具体如下:
2.8.1 增材制造骨科产品 根据产品的三维数字模型,利用增材制造工艺生产骨科植入物,主要通过连续的物理叠加,逐层增加材料生成三维实体,可实现个性化生产及精细加工骨科植入物,适用于骨及附属组织的支持、固定、替代[69]。目前商业化应用的骨科植入物产品有:①金属增材制造匹配式长段骨缺损修复体,由医用级钛合金(Ti-6AL-4V Eli)
增材制造技术制成。依据患者解剖结构进行匹配,在植入设备建造之前,外科医生通过将他/她的设计要求与工程图纸进行比较,来批准3D打印骨段的设计。该产品TC桥(TC Bridge)基于医工交互平台,提供从术前规划、医工交互、3D打印定制化设计与制造加工,最终交付完成临床手术,用于因创伤、肿瘤、感染、骨髓炎等造成的大段骨缺损重建治疗,如爱康医疗的金属增材制造匹配式长段骨缺损修复体Additive Orthopaedics、LLC公司的Additive Orthopaedics Patient Specific 3D Printed Bone Segments(骨科患者特定的3D打印骨段)。②增材制造椎间融合器,由颈椎椎间融合器和腰椎椎间融合器组成,由符合YY/T 1851标准规定的钽金属粉末材料或符合ASTM F3001标准要求的Ti-6Al-4V ELI原材料粉末,通过激光选区熔融制造技术制成。产品带有和不带有植骨窗,与脊柱内固定系统联合使用,适用于椎间融合术,如湖南华翔医疗科技有限公司的增材制造椎间融合器、强生的CONDUIT? Interbody Cages(CONDUIT?椎间融合器)、Medicrea International S.A.公司的IMPIX 3D Print cages(IMPIX 3D打印融合器)、Medicrea International S.A.公司的UNiD Patient Specific 3D printed cage(UNiD患者专用3D打印融合器)。③定制式增材制造膝关节矫形器:由股骨托、胫骨托、连接器和绑带组成。胫骨托和股骨托是根据医生提供的患者CT数据进行设计,由聚十二内酰胺通过增材制造工艺制成,连接器由铝合金6061机加工组成,绑带由尼龙丝制成。根据专业医师提供的患者数据个性化设计生产,供提出需求的医疗机构用于指定患者矫正轻中度膝关节畸形。④增材制造植入物——椎体假体:由TC4钛合金材料制成,通过电子束熔融快速成型技术建立互相连接的微孔而制成的多孔植入物,表面无着色,与脊柱内固定系统联用,用于椎体切除手术中的椎体替代。⑤金属增材制造胸腰椎融合匹配式假体系统:包括依据患者胸腰椎解剖结构进行匹配的胸腰椎融合匹配式假体以及配合组件钉扣、螺钉,通过增材制造技术,由TC4钛合金材料制成;钉扣(标准化规格)与胸腰椎融合匹配式假体配合使用,由2型超高分子质量聚乙烯材料制成;螺钉(标准化规格)与胸腰椎融合匹配式假体配合使用,由TC4钛合金材料机加工制成。需与脊柱内固定系统匹配,适用于上胸椎至下腰椎(T1-L5)因肿瘤或其它病变需行连续3个及以上节段椎体切除后的结构重建。⑥关节假体:如Microport Orthopedics,Inc.公司生产的BIOFOAM?增材制造产品,EVOLUTION? BIOFOAM胫骨平台、ADVANCE? BIOFOAM胫骨平台用于膝关节置换,DYNASTY? BIOFOAM髋臼杯用于髋关节置换,BIOFOAM?增材制造(BIOFOAM?AM)作为用作多孔涂层BIOFOAM?材料的替代品。BIOFOAM?AM将用作MicroPort Orthopedics的胫骨基体和髋臼杯的多孔涂层,原材料是符合ASTM F67的商业纯钛,其结构和孔隙率可促进骨形成。⑦髋关节假体:如Gillen Gonzales公司的Prime 和DYNASTY? Additive Manufacturing Shells Prime(Prime和DYNASTY?增材制造外杯Prime),由钛合金(Ti-6Al-4V)粉末通过增材制造工艺制成,用于髋关节置换中骨界面表面的无骨水泥固定。
2.8.2 增材制造口腔科产品 口腔修复领域是增材制造技术研究和应用重要领域之一,口腔修复体具有形态复杂、个性化特征强、需要多种材料一体化成形、功能与美学需求高等特点,这与3D打印成形原理和特点具有很高的契合度[70]。在过去5年中由于口内扫描技术的进步,3D 打印机的可及性和可打印生物材料的开发,3D 打印技术已经显著改变了牙科医学。
通常用于打印金属材料的激光选区熔化技术在口腔和活动修复体加工方面的研究与应用已经逐渐成熟,是口腔修复领域具有代表性的技术方向,光固化树脂、金属、陶瓷等3D打印修复在口腔修复临床上用于创建修复体、颌骨模型及导槽、正畸牙套和其他牙科应用[71]。通常使用生物相容性金属合金如钴铬、钛和不锈钢合金来制造牙冠、牙桥、植入体和组织义齿框架[72]。3D打印瓷修复体通常具有较低的机械特性,并且表面没有那么精细[73],牙瓷由于具有与实际牙齿相似的特征,如抗压强度、导热性、颜色耐久性和高美学价值,成为牙科/美容牙科的重中之重材料;然而,由于与传统或铣削技术创建的相同修复相比太硬,因此难以操作,成为瓷器3D打印的挑战。
随着注册审评制度的发展和完善,国内外获批注册口腔类增材制造产品日益增多,由(甲基)丙烯酸酯、光引发剂[二苯基-(2,4,6-三甲基苯甲酰)氧磷]和颜料组成,用于通过增材制造工艺(3D打印工艺)制作临时冠桥修复体,如广州黑格的增材制造用光固化临时冠桥树脂,Enlighten Materials Co.,Ltd 公司的AA temp temporary restoration 3D printing photoreactive resin(AA临时修复3D打印光反应树脂)、BB Base 3D Printing Resin for Denture Base(BB基3D打印义齿基托树脂)。
2.8.3 增材制造术前规划导板/骨模型 增材制造产品用于术前规划导板或骨模型,制造与实物具有等比例的产品,用于提供给临床医生辅助观察病患部位或手术前操练与演习,可提供术前/术中的定位、导向、评估或提供基准面,以及时发现手术方案中的问题并改进,提高手术精准性和成功率。常见的产品有:①增材制造骨模型:依据影像学设备生成的患者数据,经数据转换和三维立体重建设计后,采用高分子材料——聚乳酸,利用3D打印机等比例地将重建后的三维立体图打印为骨模型,适用于完整显示骨盆、四肢、头颅、脊柱骨骼的解剖结构及病患骨骼骨部位表面形态,产品尺寸与实物等比例,用于提供给临床医生辅助观察病患骨部位或者手术前操练与演习,如个性化增材制造骨模型。②医用骨科手术导板:作为骨科手术配套基础工具,是根据影像学数据进行三维重建后经3D打印制成。采用高分子材料[如聚乳酸、聚十二内酰胺、高分子树脂材料(双酚A二甲基丙烯酸酯、甲基丙烯酸2-羟乙酯、双甲基丙烯酸尿烷酯)等]制成,用于骨科(如骨盆、膝关节、胫骨近端、踝关节、下肢骨干、下颌骨、颈椎、胸腰椎部位)手术中定位、导向、评估或提供基准面。③定制式牙科种植用导板:常由基板和导环两部分组成,基板采用树脂材料,根据计算机辅助设计模型通过3D打印技术制成;导环可采用外科器械用不锈钢金属材料制成。导板接触人体前,用乙醇浸泡,用于牙科种植手术中假体的定位、导向等,从而辅助安装假体。④定制式3D打印人体器官模型:为临床医护人员制定手术方案和规划提供术前指导,依据影像学设备生成的患者数据,经数据转换和三维立体重建设计,利用3D打印机将重建后的三维立体图采用丙烯酸酯树脂材料打印为实体图。用于显示解剖内部结构,帮助医师立体观察患者手术部位,确认手术位置,辅助医师制定优化手术方案,选择合适的手术路径。术前对手术进行操练演习,以及时发现手术方案中的问题并改进。
2.8.4 增材制造颅颌面部产品
(1)Osteopore Inc公司根据GMP标准通过熔融沉积成型技术生产的聚己内酯支架OSTEOPORE PCL SCAFFOLD(K051093),于2006年被FDA批准用于商业销售,这是第一个用于颅面修复的3D打印设备[74],该产品作为Ⅱ类医疗器械用于颅面缺损的骨空隙。该产品由可生物降解的聚合物——聚己内酯制成,通过水解在体内完全降解和再吸收,然后由人体代谢,该聚合物在24个月内被身体完全吸收。
(2)2011年,Oxford Performance Materials,LLC(美国牛津高性能材料公司,OPM)宣布推出OSTEOFAB PATIENT SPECIFIC CRANIAL DEVICE(骨肉瘤患者专用颅骨装置,510K号:K121818),这是一种通过选择性激光烧结由聚醚醚酮制成的患者专用增材制造颅骨植入物,它通过510 K途径获得了FDA批准为Ⅱ类医疗器械,OsteoFab患者专用颅骨装置(OPSCD)为每位患者单独构建,以纠正颅骨缺陷。该装置使用患者的CT成像数据和计算机辅助设计来确定每个植入物的尺寸,旨在替代颅骨中的骨空隙。该公司又获得了两项510 K FDA批准,用于其他聚醚醚酮患者专用选择性激光烧结制造的用于面部骨骼的OSTEOFAB PATIENT SPECIFIC FACIAL DEVICE(骨肉瘤患者专用面部装置,510K号:K133809)和椎骨的SpineFab Vertebral Body Replacement(VBR)System(SpineFab椎体置换系统,510K号:K142005)医疗设备。
OsteoFab患者专用面部设备(OPSFD)是为每位患者单独构建的,由聚醚酮酮聚合物制成,由激光烧结机制造,使用患者的CT成像数据和计算机辅助设计来确定每个植入物的尺寸。OsteoFab患者专用面部设备有多种配置,这取决于应用程序的几何形状。OsteoFab患者专用面部设备呈椭圆形,对于单个患者的形状和尺寸在以下规格范围内变化:最大直径为20 cm,最小厚度为1 mm,最大厚度为10 mm,最大开孔密度为25%,最小建孔直径为3 mm,最大建孔直径为5 mm,从建孔边缘到设备边缘的最小距离为15 mm。OsteoFab患者专用面部设备通过商用固定系统附着在天然骨上,是一种永久性植入物。
SpinFab? VBR假体是一种非定制植入物,由48种不同的变体组成,每一种都有6种不同的配置(小平行、中平行、大平行、小前凸、中前凸和大前凸),每种配置的唯一区别是高度。该装置使用增材制造的聚醚酮酮聚合物制造,并含有钽射线照相标记物。植入物有一个开放的轴,允许同种异体移植物或自体移植物的放置。这种植入物还有其他几个特点:凹槽有助于植入物的插入,钽标记物允许轻松的放射成像可视化,旨在与椎体终板接合以实现体内稳定。
(3)Materialise公司的TruMatch CMF Titanium 3D Printed Implant System (TruMatch CMF钛合金3D打印假体系统)可用于颌面骨骼、面中部、下颌骨及颏部的骨再定位、固定及重建。TruMatch CMF钛3D打印种植系统提供了一种个性化钢板和个性化截骨及钻孔附件的解决方案,可将手术计划准确地传递到手术室,用于青少年(12-21岁)和成年人的颌面骨骼、中脸、下颌骨和下巴的骨重新定位、固定和重建。
综上可知,对临床上需要个性化定制产品的患者,已有大量产品可生产并临床使用,如WONG等[75]报道了进行肿瘤切除后,根据患者其骨盆缺损形状和生物力学分析设计并打印植入物,进行个性化治疗,患者术后髋关节恢复良好;MCALLISTER等[76]在引导切割手术中通过增材制造技术为患者个性化定制了用于切割畸形颌骨的截骨导板,外科医生根据导板确定截骨切割的最佳方向,并更好地预测术后骨骼上下颌的关系。