中国组织工程研究 ›› 2020, Vol. 24 ›› Issue (31): 4986-4993.doi: 10.3969/j.issn.2095-4344.2141
• 干细胞移植 stem cell transplantation • 上一篇 下一篇
预处理不含抗胸腺细胞球蛋白非血缘脐血移植治疗急性髓系白血病和急性淋巴细胞白血病306例随访评价
张旭晗1,王 丽2,汤宝林1,皖 湘1,姚 雯1,宋闿迪1,孙自敏1
- 中国科学技术大学附属第一医院(安徽省立医院),1血液科, 2康复医学科,安徽省合肥市 230036
-
收稿日期:2020-02-29修回日期:2020-03-06接受日期:2020-04-03出版日期:2020-11-08发布日期:2020-09-03 -
通讯作者:孙自敏,主任医师,教授,中国科学技术大学附属第一医院(安徽省立医院),安徽省合肥市 230001 -
作者简介:张旭晗,男,1983年生,汉族,2010年安徽医科大学毕业,硕士,主治医师,主要从事造血干细胞移植并发症及急性白血病分层诊治的研究。 -
基金资助:国家自然科学基金(面上项目) (81470350)
Pretreatment of unrelated umbilical cord blood transplantation without antithymocyte globulin for the treatment of acute myeloid leukemia and acute lymphoblastic leukemia: follow-up evaluation of 306 cases
Zhang Xuhan1, Wang Li2, Tang Baolin1, Wan Xiang1, Yao Wen1, Song Kaidi1, Sun Zimin1
- 1Department of Hematology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China; 2Department of Rehabilitation Medicine, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230036, Anhui Province, China
-
Received:2020-02-29Revised:2020-03-06Accepted:2020-04-03Online:2020-11-08Published:2020-09-03 -
Contact:Sun Zimin, Chief physician, Professor, Department of Hematology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China -
About author:Zhang Xuhan, Master, Attending physician, Department of Hematology, The First Affiliated Hospital of USCT, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China -
Supported by:the National Natural Science Foundation of Chin, No. 81470350
摘要:
文题释义:
非血缘脐血造血干细胞移植:指利用公共脐脐血库寻找到HLA匹配的非血缘脐血进行干细胞移植。回输脐血之前需要经过以化疗或者放疗为基础的清髓性预处理,为脐血干细胞的归巢提供龛位。
移植物抗宿主病:指由于移植后供者T淋巴细胞介导的以受者体细胞为目标的细胞毒攻击,其中皮肤、肝及肠道是主要的靶目标。急性移植物抗宿主病的发生率为30%-45%,慢性者发生率低于急性。
背景:脐血造血干细胞移植作为急性白血病的根治手段,应用越来越广泛,但是其在不同白血病的治疗效果尚无对比,通过分疾病的疗效对比,可指导不同患者进行移植方式的选择。
目的:对比分析脐血造血干细胞移植治疗急性髓系白血病和急性淋巴细胞白血病的疗效差异。
方法:回顾性分析接受非血缘脐血造血干细胞移植治疗的306例急性白血病患者的临床资料,其中急性淋巴细胞白血病194例,急性髓系白血病112例。所有患者均接受不含抗胸腺细胞球蛋白的清髓性预处理方案,预防移植物抗宿主病为环孢素联合吗替麦考酚酯。
结果与结论:①除了急性淋巴细胞白血病移植后的复发率比急性髓系白血病略高以外,两组患者在接受非血缘脐血移植术后的疗效基本一致;②在青少年和年轻成人组(年龄15-39岁),急性髓细胞白血病的中性粒细胞和血小板植入速率均快于急性淋巴白血病白血病,其中CD34+细胞数和预处理方案是针对中性粒细胞植入的独立影响因素,而CD34+细胞数同时也是针对血小板植入的独立影响因素,在该年龄组,急性淋巴细胞白血病患者移植后的复发率依然高于急性髓系白血病,其中慢性移植物抗宿主病是独立影响因素;③移植后免疫重建检测提示,脐血移植后4个月时急性髓系白血病患者的脐血CD8+T细胞重建要优于急性淋巴细胞白血病患者;④上述数据说明,预处理不含抗胸腺细胞球蛋白的非血缘脐血移植对于急性淋巴细胞白血病和急性髓系白血病均有良好的疗效。中国科学技术大学附属第一医院(安徽省立医院)血液科具有干细胞移植资质。
orcid: 0000-0001-9395-0133(Zhang Xuhan)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
张旭晗, 王 丽, 汤宝林, 皖 湘, 姚 雯, 宋闿迪, 孙自敏. 预处理不含抗胸腺细胞球蛋白非血缘脐血移植治疗急性髓系白血病和急性淋巴细胞白血病306例随访评价[J]. 中国组织工程研究, 2020, 24(31): 4986-4993.
Zhang Xuhan, Wang Li, Tang Baolin, Wan Xiang, Yao Wen, Song Kaidi, Sun Zimin. Pretreatment of unrelated umbilical cord blood transplantation without antithymocyte globulin for the treatment of acute myeloid leukemia and acute lymphoblastic leukemia: follow-up evaluation of 306 cases[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(31): 4986-4993.
Patient and transplant characteristics
Patients and transplant characteristics are summarized in Table 1. Among 306 patients, 112 (36.6%) received transplantation for AML and 194 (63.1%) for ALL. Median age at UCBT was 13.87 years (range, 1-64 years) for patients with AML and 11.89 years (range, 1-50 years) for those with ALL. In the AML group, 65 patients (58%) were transplanted in CR1, 57 (18.7%) in CR2, and 25 (22.3%) in advanced disease status. For the ALL group, 103 patients (53%) were transplanted in CR1, 57 (29.4%) in CR2, and 24 (12.4%) in advanced disease status. Overall, 248 patients (81%) had high-risk disease at diagnosis. Among all patients, 31 (10.1%) received UCB that was 6/6 HLA matched, 152 (49.7%) with a 5/6 HLA match, and 123 (40.2%) with a 4/6 HLA match. A total 118 patients (38.5%) had matched donor ABO compatibility, 101 (33%) had major ABO mismatch, and 87 (28.4%) had minor ABO mismatch. There were 203 patients (66.3% of the entire population, of which 69 had AML and 134 had ALL) who received a conditioning regimen of BUCY2 in combination with fludarabine. Another 103 patients (33.3% of the total population, of which 43 had AML and 60 with ALL) received a conditioning regimen of total body irradiation cyclophosphamide plus cytarabine. For patients with AML, the median total nucleated cell dose and CD34+ cell dose was 4.25 × 107/kg (range: 1.71-17.27 × 107/kg) and 2.18 × 105/kg (range: 0.45-10.55 × 105/kg), respectively. For ALL patients, the median total nucleated cell dose and CD34+ cell dose was 4.22 × 107/kg (range: 1.69-13.5 × 107/kg) and 2.25 × 105/kg (range, 0.4-8.53 × 105/kg), respectively.

Outcomes of transplantation
For the total population of patients with AML and ALL, the engraftment of neutrophil and platelet were similar. The 30-day cumulative incidences of neutrophil engraftment after UCBT in the AML and ALL groups were 95.5% (95% CI: 89.1-98.2) and 94.8% (95% CI: 90.5-97.2), respectively (P=0.718). The median time to neutrophil recovery was 17 (range, 12-30) days and 16 (range, 10-31) days, respectively (Table 2). Fourteen patients did not engraft after UCBT (AML: 5 and ALL: 9); four of those patients—all of whom had ALL-died from severe infection and cerebral hemorrhage (two patients each). The 120-day cumulative incidences of platelet engraftment for AML and ALL was 83.9% (95% CI: 75.6-89.7) and 82.5% (95%CI: 76.3-87.2; P=0.733) and the median time to platelet recovery was 36 (range, 17-121) and 36 (range, 11-196) days, respectively.
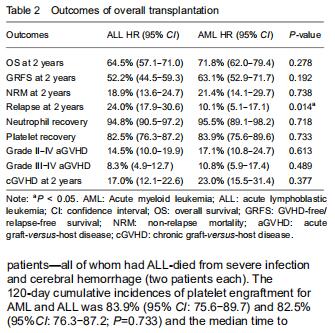
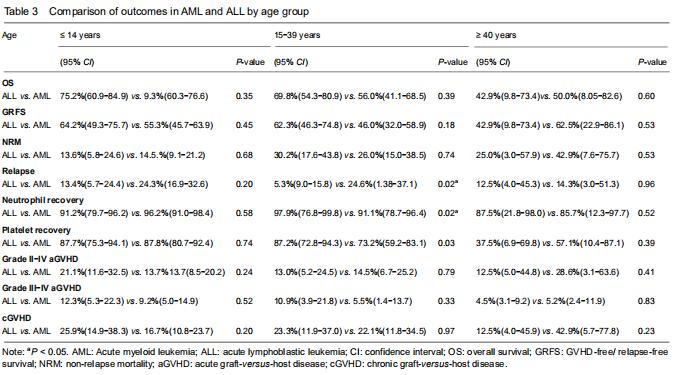
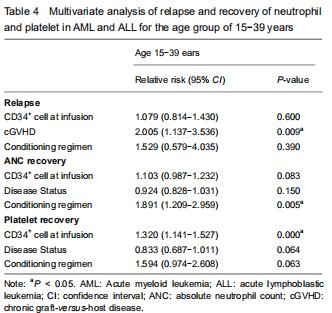
With respect to age groups (≤ 14 years, 15-39 years, ≥ 40 years) (Table 3), adolescents and young adults group had higher engraftment rates for AML than ALL. The 30-day cumulative incidence of neutrophil recovery was higher in patients with AML than in those with ALL (97.9%, 95% CI: 76.8-99.8, vs. 91.1%, 95% CI: 78.7-96.4, P=0.02). In multivariate analysis, CD34+ cell dose and conditioning regimen were independent factors influencing neutrophil recovery. The cumulative incidence of platelet recovery on day 120 was also higher in AML than in ALL (87.2%, 95% CI: 72.8-94.3, vs. 73.2%, 95% CI: 59.2-83.1; P=0.03). In multivariate analysis, CD34+ cell dose was the only independent factor influencing platelet recovery (Table 4). There was no difference between AML and ALL in the groups of children and old adults.
Incidences of acute and chronic GVHD
For the total population of patients with AML and ALL, the cumulative incidences of acute GVHD (grade II-IV and grade III-IV) at 100 days were similar (grades II-IV: AML, 17.1% (95% CI: 10.8-24.7) and ALL, 14.5% (95% CI: 10.8-24.7), P=0.613; grades III-IV: AML, 11% (95% CI: 5.9-17.4) and children, 8% (95% CI: 4.9-12.7), P=0.489). The 2-years cumulative incidences of cGVHD in patients with AML and ALL were also similar (23%, (95% CI: 15.5-31.4) and 17%, (95% CI: 12.1-22.6, P=0.377). With grouping by age, cumulative incidences of aGVHD and cGVHD were still similar between AML and ALL in children, adolescents and young adults, and older adults.
Relapse and non-relapse mortality, overall survival, and GRFS
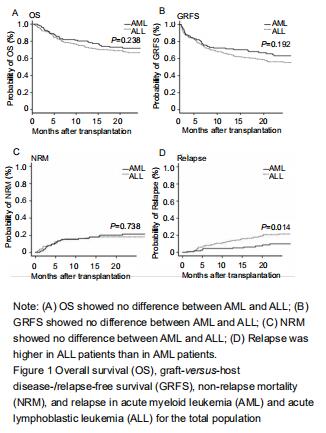
Fifty-four patients had relapse after UCBT (11 with AML and 43 with ALL). The cumulative incidence for relapse at 2 years in patients with ALL was higher than with AML (24%, 95% CI: 17.9-30.6, vs. 10.1% 95% CI: 5.1-17.1, P=0.01) (Figure 1). The median time to relapse was 12.7 (range, 1.1-59.6) months among AML and 10 (range, 1.5-34.8) months among ALL. In the analysis of age-group, both cumulative incidence of relapse and median time to relapse were significantly different between AML and ALL in adolescents and young adults group (relapse: 25%, 95% CI: 1.4-37.1, vs. 9%, 95% CI: 5.3-15.8, P=0.02); median time: 20.4 (range, 18.6-59.6) months vs. 8.1 (range, 2.8-17.7) months). There were no differences between AML and ALL in both children group and old adult group. In multivariate analysis, cGVHD was an independent factor influencing relapse.
Sixty patients died without relapse, 23 with AML and 37 with ALL. The median time to non-relapse mortality in AML and ALL was 301 (range: 45-1 044) days and 121 (range: 3-1 392) days, respectively. The cumulative incidence of non-relapse mortality at 2 years was 21.4%(95% CI: 14.1-29.7) for AML and 18.1%(95% CI: 13.0-23.9) for ALL (P=0.738). Causes of death included pulmonary infection (n=30), hemorrhage (n=4), multiorgan failure (n=22), and other causes (n=4). There was no significant difference in non-relapse mortality between AML and ALL by age groups.
The median follow-up duration was 21.6 (range, 3-80.7) months. Overall survival at 2 years in AML was similar to that in ALL (72%, 95% CI: 62-79.4, vs. 66%, 95% CI: 59.5-70.3, P=0.263). There was also no difference in overall survival between AML and ALL by age groups. The GRFS at 2 years in AML and ALL was 63.1% (95% CI: 52.9-71.7) and 56.2% (95% CI: 48.7-63.0), respectively (P=0.192). GRFS also showed no difference between AML and ALL by age groups.
Immune reconstitution
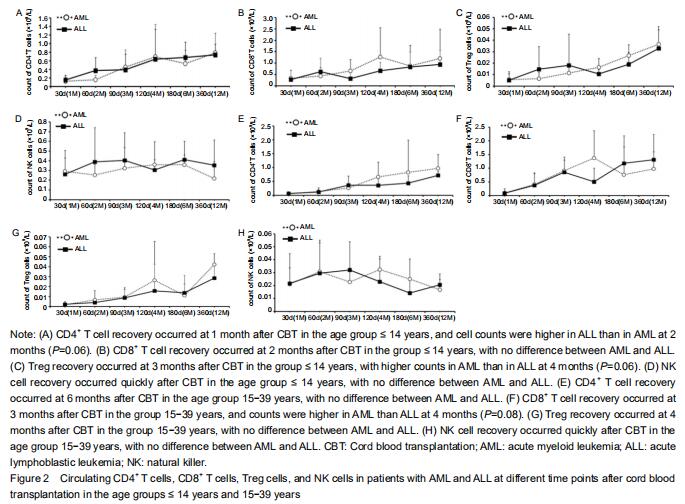
Considering the effect of age on the development of the immune system, the immune reconstitution was compared among age groups. In the comparison of outcomes between AML and ALL, we analyzed age groups ≤ 14 years and 15-39 years with respect to CD4+ T cells, CD8+ T cells, natural killer cells, and Treg cells at 1, 2, 3, 4,6, 12, and 18 months after CBT (Figure 2).
The results showed that CD4+, CD8+, and Treg cells recovered with time after CBT whereas natural killer cells recovered more quickly (within 1 month) in patients in both age groups. In the group ≤ 14 years, CD4+ T cell counts in AML were lower than in ALL at 2 months after CBT whereas Treg cell counts in AML were higher than in ALL at 4 months post CBT. There was no difference seen in CD8+ and natural killer cells between AML and ALL. The proportion of CD4/CD8 T cells was lowest at 2 months and recovered at 4 months after CBT.
In the age group 15-39 years, CD8+ T cell counts in AML at 4 months post CBT were higher than in ALL whereas CD4+, Treg, and natural killer cells showed no difference between AML and ALL. The proportion of CD4+/CD8+ T cells was lowest at 3 months and recovered at 12 months post CBT.
| [1] TANAKA M, MIYAMURA K, TERAKURA S, et al. Comparison of cord blood transplantation with unrelated bone marrow transplantation in patients older than fifty years. Biol Blood Marrow Transplant. 2015;21(3):517-525. [2] RUGGERI A, LABOPIN M, SANZ G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9): 1891-900. [3] PAGE KM, LABOPIN M, RUGGERI A, et al. Factors associated with long-term risk of relapse after unrelated cord blood transplantation in children with acute lymphoblastic leukemia in remission. Biol Blood Marrow Transplant. 2017;23(8):1350-1358. [4] DALLE JH, BALDUZZI A, BADER P, et al. Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 International-BFM studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant. 2018;24(9):1848-1855. [5] TANG X, CHEN J, FANG J, et al. Similar outcomes of allogeneic hematopoietic cell transplantation from unrelated donor and umbilical cord blood vs. sibling donor for pediatric acute myeloid leukemia: multicenter experience in China. Pediatr Transplant. 2015;19(4):413-421. [6] RUGGERI A,VOLT F, LOCATELLI F, et al. Unrelated cord blood transplantation for acute leukemia diagnosed in the first year of life:outcomes and risk factor analysis. Biol Blood Marrow Transplant. 2017;23(1):96-102. [7] RAIOLA AM, DOMINIETTO A, DI GRAZIA C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10): 1573-1579. [8] RUGGERI A, CICERI F, GLUCKMAN E, et al. Alternative donors hematopoietic stem cells transplantation for adults with acute myeloid leukemia:Umbilical cord blood or haploidentical donors? Best Pract Res Clin Haematol. 2010;23(2):207-216. [9] TONG J, XUAN L, SUN Y, et al. Umbilical cord blood transplantation without Antithymocyte Globulin results in similar survival but better quality of life compared with unrelated peripheral blood stem cell transplantation for the treatment of acute leukemia-a retrospective study in China. Biol Blood Marrow Transplant. 2017;23(9):1541-1548. [10] TRAMA A, BOTTA L, FOSCHI R, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000-07:population-based data form EUROCARE-5. Lancet Oncol. 2016;17(7):896-906. [11] ZHENG C, TANG B, ZHU X, et al. Pre-engraftment bloodstream infections in acute leukemia patients undergoing unrelated cord blood transplantation following intensified myeloablative conditioning without ATG. Ann Hematol. 2017;96(1):115-124. [12] SUN Z, LIU H, LUO C, et al. Better outcomes of modified myeloablative conditioning without antithymocyte globulin versus myeloablative conditioning in cord blood transplantation for hematological maliganacies: a retrospective (development) and a prospective (validation) study. Int J Cancer. 2018;143(3):699-708. [13] PRZEPIORKA D, CHAN KW, CHAMPLIN RE, et al. Prevention of graft-versus-host disease with anti-CD5 ricin A chain immunotoxin after CD3-depleted HLA-nonidentical marrow transplantation in pediatric leukemia patients. Bone Marrow Transplant. 1995;16:737-741. [14] FLOWERS ME, KANSU E, SULLIVAN KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13: 1091-1112. [15] HUGUET F, LEGUAY T, RAFFOUX E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative actute lymphoblastic leukemia: the GRAALLL-2003 study. J Clin Oncol. 2009;27:911-918. [16] DOHNER H, ESTEY E, GRIMWADE D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [17] GRAY RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988,16:1141-1154. [18] KANDA Y. Investigation of the freely available easy-to-use software ‘ESR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [19] BOROWITZ MJ, WOOD BL, DEVIDAS M, et al. Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children’s Oncology Group study AALL0232. Blood. 2015;126:964-971. [20] ESPARZA SD, SAKAMOTO KM. Topics in pediatric leukemia-acute lymphoblastic leukemia. MedGenMed. 2005;7:23. [21] BAKHTIAR S, SALZMANN-MANRIQUE E, Hutter M, et al. AlloHSCT in pediatric ALL and AML in complete remission: improvement over time impacted by accrediatation? Bone Marrow Transplant. 2019;54(5):737-745. [22] ARAKAWA Y, KATO M, KOH K, et al. Unrelated cord blood and bone marrow transplantation in pediatric leukemia. Pediatric Int. 2014;56(4): 647-650. [23] SIEGEL RL, MILLER KD, JEMAL A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [24] GIANNOTTI F, LABOPIN M, SHOUVAL R, et al. Haploidentical transplantation is associated with better overall survival when compared to single cord blood transplantation: an EBMT-Eurocord study of acute leukemia patients conditioned with thiotepa, busulfan, and fludarabine. J Hematol Oncol. 2018;11(1):110. [25] KONUMA T, TSUKADA N, KANDA J, et al. Comparison of transplant outcomes from matched sibling bone marrow or peripheral blood stem cell and unrelated cord blood in patients 50 years or older. Am J Hematol. 2016, 91(5):E284-E292. [26] LOU X, ZHAO C, CHEN H. Unrelated donor umbilical cord blood transplant versus unrelated hematopoietic stem cell transplant in patients with acute leukemia: a meta-analysis and systematic review. Blood Rev. 2018;32(3): 192-202. [27] LAWITSCHKA A, PETERS C. Long-term effects of myeloablative allogeneic hematopoietic stem cell transplantation in pediatric patients with acute lymphoblastic leukemia. Curr Oncol Rep. 2018;20(9):74. [28] NISHIMOTO T, SATOH T, TAKEUCHI T, et al. Critical role of CD4(+)CD25(+) regulatory T cells in preventing murine autoantibody-mediated thrombocytopenia. Exp Hematol. 2012;40:279-289. [29] AKAHOSHI Y, KANDA J, GOMYO A, et al. Risk factors and impact of secondary failure of platelet recovery after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2016;22(9):1678-1683. [30] WARLICK ED, PEFFAULT DE LATOUR R, SHANLEY R, et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Bio Blood Marrow Transplant. 2015;21(2):357-363. [31] YANADA M, KUROSAWA S, YAMAGUCHI T, et al. Effect of related donor availability on outcome of AML in the context of related and unrelated hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48: 390-395. [32] JINDRA P, MUZIK J, INDRAK K, et al. The outcome of allogeneic HSCT in older AML patients is determined by disease biology and not by the donor type: an analysis of 96 allografted AML patients ≥50 years from the Czech acute leukaemia clinical register (alert). Neoplasma. 2013;60: 576-583. [33] MO XD, TANG BL, ZHANG XH, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high-risk acute lymphoblastic leukemia. Int J Cancer. 2016;139(9):2106-2115. [34] BENADIBA J, ANSARI M, KRAJINOVIC M, et al. Pharmacokinetics-adapted Busulfan-based myeloablative conditioning before unrelated umbilical cord blood transplantation for myeloid malignancies in children. PLoS One. 2018;13(4):e0193862. [35] RANTI J, KURKI S, SALMENNIEMI U, et al. Early CD8+-recovery independently predicts low probability of disease relapse but also associates with severe GVHD after allogeneic HSCT. PLoS One. 2018; 13(9):e0204136. [36] HUTTUNEN P, TASKINEN M, SIITONEN S, et al. Saarinen-Pihkala UM. Impact of very early CD4(+)/CD8(+) T cell counts on the occurrence of acute graft-versus-host disease and NK cell counts on outcome after pediatric allogeneic hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2015;62(3):522-528. [37] HILLHOUSE EE, THIANT S, MOUTUOU MM, et al. Double negative T cells levels correlate chronic graft-versus-host-disease severity. Biol Blood Marrow Transplant. 2019;25(1):19-25. [38] CUPIT MC, DUNCAN C, SAVANI BN, et al. Childhood to adult transition and long-term follow-up after blood and marrow transplantation. Bone Marrow Transplant. 2016;51(2):176-181. [39] WOOD WA, LEE SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803-5815. [40] NSIR SS, GIRI S, NUNNERY S, et al. Outcomes of adolescents and young adults compared with pediatric patients with acute myeloid and promyelocytic leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:126-132. [41] PULEWKA K, WOLFF D, HERZBERG PY, et al. Physical and psychosocial aspects of adolescent and young adults after allogeneic hematopoietic stem cell transplantation: results form a prospective multicenter trial. J Cancer Res Clin Oncol. 2017;143(8):1613-1619. |
| [1] | 林清凡, 解一新, 陈婉清, 叶振忠, 陈幼芳. 人胎盘源间充质干细胞条件培养液可上调缺氧状态下BeWo细胞活力和紧密连接因子的表达[J]. 中国组织工程研究, 2021, 25(在线): 4970-4975. |
| [2] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [3] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [4] | 王正东, 黄 娜, 陈婧娴, 郑作兵, 胡鑫宇, 李 梅, 苏 晓, 苏学森, 颜 南. 丁酸钠抑制氟中毒可诱导小胶质细胞活化及炎症因子表达增多[J]. 中国组织工程研究, 2021, 25(7): 1075-1080. |
| [5] | 汪显耀, 关亚琳, 刘忠山. 提高间充质干细胞治疗难愈性创面的策略[J]. 中国组织工程研究, 2021, 25(7): 1081-1087. |
| [6] | 万 然, 史 旭, 刘京松, 王岩松. 间充质干细胞分泌组治疗脊髓损伤的研究进展[J]. 中国组织工程研究, 2021, 25(7): 1088-1095. |
| [7] | 廖成成, 安家兴, 谭张雪, 王 倩, 刘建国. 口腔鳞状细胞癌干细胞的治疗靶点及应用前景[J]. 中国组织工程研究, 2021, 25(7): 1096-1103. |
| [8] | 谢文佳, 夏天娇, 周卿云, 刘羽佳, 顾小萍. 小胶质细胞介导神经元损伤在神经退行性疾病中的作用[J]. 中国组织工程研究, 2021, 25(7): 1109-1115. |
| [9] | 李珊珊, 郭笑霄, 尤 冉, 杨秀芬, 赵 露, 陈 曦, 王艳玲. 感光细胞替代治疗视网膜变性疾病[J]. 中国组织工程研究, 2021, 25(7): 1116-1121. |
| [10] | 焦 慧, 张一宁, 宋雨晴, 林 宇, 王秀丽. 乳腺癌类器官研究进展及临床应用前景[J]. 中国组织工程研究, 2021, 25(7): 1122-1128. |
| [11] | 王诗琦, 张金生. 中医药调控缺血缺氧微环境对骨髓间充质干细胞增殖、分化及衰老的影响[J]. 中国组织工程研究, 2021, 25(7): 1129-1134. |
| [12] | 曾燕华, 郝延磊. 许旺细胞体外培养及纯化的系统性综述[J]. 中国组织工程研究, 2021, 25(7): 1135-1141. |
| [13] | 孔德胜, 何晶晶, 冯宝峰, 郭瑞云, Asiamah Ernest Amponsah, 吕 飞, 张舒涵, 张晓琳, 马 隽, 崔慧先. 间充质干细胞修复大动物模型脊髓损伤疗效评价的Meta分析[J]. 中国组织工程研究, 2021, 25(7): 1142-1148. |
| [14] | 侯婧瑛, 于萌蕾, 郭天柱, 龙会宝, 吴 浩. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究, 2021, 25(7): 985-990. |
| [15] | 史洋洋, 秦英飞, 吴福玲, 何 潇, 张雪静. 胎盘间充质干细胞预处理预防小鼠毛细支气管炎[J]. 中国组织工程研究, 2021, 25(7): 991-995. |
In recent years, unrelated cord blood transplantation (UCBT) has become increasingly used in high-risk acute leukemia. However, investigations of the outcomes for acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) after single-unit UCBT have mostly focused on comparisons with other types of allogeneic hematopoietic stem cell transplantation (allo-HSCT), such as matched related sibling donor, matched or unrelated related donor, or haplo donor[1,2].
Compared with a suitable sibling donor, cord blood transplantation (CBT) can result in similar or even reduced incidence of relapse and graft-versus-host disease (GVHD) for pediatric ALL with a very high risk of relapse. CBT also has survival rates that are similar to those with suitable sibling donor in cases of high-risk pediatric AML, such as primary refractory AML or later than the first complete remission (CR1)[3-5]. Thus, there was a previous study compared the outcomes of single-unit CBT between AML and ALL in a restricted age group in the first year of life[6]. In comparison with other source donors, the outcomes of CBT in adults are controversial. In an earlier study, the 2-year incidence of relapse, transplantation-related mortality and leukemia-free survival of CBT in adults with acute leukemia was comparable with that of human leukocyte antigen (HLA)-matched unrelated hematopoietic stem cell transplantation (bone marrow or peripheral blood)[7]. Subsequent research indicated that CBT in adults was associated with delayed neutrophil recovery and higher incidence of acute GVHD (aGVHD)[8]. Yet in a previous study at our center, CBT without antithymocyte globulin in the conditioning regimen resulted in better outcomes than unrelated peripheral blood stem cell transplantation[9]. Therefore, the outcomes of CBT in adults with acute leukemia still depend on the center experience. However, there are no comparative data on the outcomes of adults with AML and ALL after single-unit CBT.
For choosing the better style of transplantation, we were interested in determining whether AML and ALL have similar outcomes among different age groups after single-unit CBT using the same conditioning regimen, such as myeloablative conditioning without antithymocyte globulin. As the biological differences of acute leukemia between adolescents and young adults (aged 15-39 years) and adults has been confirmed, more and more studies have focused on the outcomes of adolescents and young adults, in whom treatment strategies have remained controversial until now[10]. Therefore, we compared the outcomes of AML and ALL after single-unit CBT using myeloablative conditioning without antithymocyte globulin among different groups based on age, in children, adolescents and young adults, and adults. Different to the findings of previous research, our results add new data regarding immune reconstitution.
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
Design
Retrospective study.
Time and setting
This work was conducted at First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital) from November 2011 to July 2018.
Participants
This retrospective registry-based study included 112 patients with AML and 194 with ALL in First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital), between November 2011 and July 2018. Considering the difference in the age at diagnosis between AML and ALL, we used grouping by age to minimize the impact of this difference. The age groups were as follows: ≤ 14, 15-39, and ≥ 40 years.
All patients were treated according to our protocol, which is registered at www.chictr.org.cn (# ChiCTR-ONRC- 11001430). Written informed consent was obtained from all patients or their guardians prior to the start of conditioning. The transplant protocol was approved by the Ethics Committee of Anhui Provincial Hospital (approval No. 2011-089) on March 31, 2011.
Inclusion criteria
(1) All patients were diagnosed as having ALL or AML. (2) All patients had a suitable cord blood for transplantation. (3) All patients agreed to receive CBT.
Exclusion criteria
There were (1) impaired heart and renal function; (2) uncontrolled mental disease; (3) refused to receive CBT.
Methods
All patients who received single-unit CBT possessed suitable criteria for transplantation, as previously reported[11]. CB unit selection was based on the total nucleated cell and CD34+ cell number among 4/6 to 6/6 HLA-matched units. All cord blood units were obtained through the Chinese Cord Blood Band Network. The conditioning regimen for patients with both AML and ALL was myeloablative conditioning. For patients aged ≤ 14 years or those receiving radiotherapy before transplantation, the conditioning regimen was based on BUCY2 (busulfan 0.8 mg/kg every 6 hours for 4 days, and cyclophosphamide 60 mg/kg daily for 2 days), plus fludarabine (30 mg/m2 daily for 4 days). For patients ≥ 14 years old or those with no remission after relapse or primary induction failure, the conditioning regimen was based on total body irradiation cyclophosphamide (total body irradiation 12 Gy given in 4 fractions, and cyclophosphamide 60 mg/kg daily for 2 days), plus high-dose cytarabine (2.0 g/m2, every 12 hours for 2 days). Granulocyte colony-stimulating factor was injected subcutaneously at 5 μg/kg daily beginning 6 days after stem cell infusion. The GVHD prophylaxis regimen was a combination of cyclosporine A and mycophenolate mofetil, as previously described[12]. All laboratory assessments were done as part of clinical care. To evaluate immune reconstitution, peripheral blood samples were obtained and tested 30, 60, 90, 120, 180, and 360 days after UCBT.
Outcomes
The endpoints included GVHD-free/relapse-free survival (GRFS), time to neutrophil and platelet recovery, incidence of non-relapse mortality, relapse, and acute and chronic GVHD (aGVHD and cGVHD, respectively).
Overall survival was calculated from the date of transplantation until death or the last observation alive. The GRFS endpoint was defined as grade III-IV aGVHD, extensive GVHD, relapse, or death. Neutrophil engraftment was defined as absolute neutrophil count > 0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as platelet count ≥ 20 × 109 L-1 without platelet transfusion for 7 days[13]. Both aGVHD and cGVHD were graded according to previously published criteria[14]. Non-relapse mortality was defined as death without prior relapse. The primary endpoints include GRFS, overall survival, non-relapse mortality and relapse. The secondary endpoints include GVHD and neutrophil and platelet recovery.
Patients had high risk factors at first diagnosis: ALL with adverse cytogenetics or molecular abnormalities [Ph+ chromosome (BCR-ABL positive), hypodiploidy, 11q23 abnormalities (MLL rearrangements), or with a high level of minimal residual disease (1% or more after completion of 6 weeks of induction therapy)]; AML with adverse cytogenetics or molecular abnormalities [t(6;9)(p23;q34.1) or t(v;11q23.3),t(9;22)(q34.1;q11.2), inv(3)(q21.3q26.6),-5 or del(5q),-7,-17/abn(17p), complex karyotype, normal cytogenetics with FLT3-ITD mutation], or prior history of myelodysplastic syndrome[15-16].
Statistical analysis
Patient- and transplant-related variables were compared between the two patient groups (AML or ALL) using the chi-square statistic for categorical variables and the Mann-Whitney test for continuous variables. Continuous variables were represented by median and categorical variables were represented by proportion. Variables considered in the analysis included patients’ age at transplantation, donor/recipient sex, disease status at transplantation (patients were divided according to the 1st remission period, the 2nd remission period, > 2nd remission, and advanced disease), HLA match, ABO compatibility, type of conditioning (total body irradiation based or BUCY2 based), total nucleated cell dose, and CD34+ cell dose. Cumulative incidence functions were used in a competing risk setting, with relapse treated as a competing event to calculate non-relapse mortality probabilities, and with death from any cause as a competing risk for GVHD, engraftment, and relapse. Probabilities of GRFS and overall survival were calculated using Kaplan-Meier estimates. Univariate analyses were performed using Gray’s test for cumulative incidence functions, and log-rank test for GRFS and overall survival[17]. Baseline variables that were considered clinically relevant or that showed a univariate relationship with outcome were entered into multivariable model. Variables for inclusion were carefully chosen, given the number of events available, to ensure parsimony of the final model. Candidate variables with a P-value < 0.2 on univariate analysis were included in the multivariable model. CD4+ T cells, CD8+ T cells, regulatory T cells (Tregs), and natural killer cells were compared between AML and ALL using the Mann-Whitney U-test and Kruskal-Wallis test. Statistical analyses were performed with EZR, version 1.37 software (Saitama Medical Center, Jichi Medical University, Japan)[18], which is a graphical user interface for R version 3.0.2 software (The R Project for Statistical Computing, Vienna, Austria). A value of P < 0.05 was considered statistically significant.
Although the guidelines of the National Comprehensive Cancer Network have highlighted that chronological age is a poor surrogate for suitable therapy, most clinical research has still been based on age groups when considering the biological difference between AML and ALL, which have at different ages of onset. In light of the differences in chemotherapy between AML and ALL, previous studies have not compared the two diseases in one therapy but rather considered them as independent factors with respect to the outcomes of therapy[15, 19]. Even in HSCT therapy, where both AML and ALL with the same risk level have the same conditioning regimens and GVHD prophylaxis, few studies have compared these two diseases in one type of HSCT. Among the various HSCT therapies, CBT is widely used in children with acute leukemia, especially pediatric ALL, which accounts for the largest proportion of pediatric leukemia; however, CBT is not recommended in adults as the first choice because of the limited CB dose[20]. In contrast, the median age of onset in AML is 67 years, with 54% of patients aged 65 years or older. Thus, there are more patients with AML undergoing transplantation than those with ALL, with CBT representing a considerable proportion of these transplants[21]. In the present study, grouping by age was used to eliminate the biological difference between AML and ALL. As compared with previous reports on single-unit CBT in AML and ALL, we conducted a systematic study and analysis to answer our research question regarding differences in outcomes of AML and ALL after single-unit CBT using myeloablative conditioning without antithymocyte globulin. For the comparison by grouping of age, we could recommend CBT for the suitable patients with AML or ALL.
Several studies have indicated that pediatric AML and ALL show no difference in survival after CBT[22-23]. In studies with a restricted age (< 1 year) for the study population, it appears that patients with AML have better survival than those with ALL, with GRFS at 4 years of 40% and 66%, respectively[9]. The present study showed that childhood acute leukemia had different outcomes after the same type of HSCT, in contrast to previous studies. In this study, the GRFS and overall survival after CBT in children group showed no differences between AML and ALL. Compared with published research with restricted age groups, there were fewer patients aged < 1 year in the age group ≤ 14 years in this study. Thus, the difference in GRFS between pediatric AML and ALL may only for single-unit CBT at a very young age but not for other types of HSCT at other ages.
There are several studies showing delayed engraftment of single-unit CBT, in contrast with other types of HSCT, such as unrelated peripheral blood stem cell transplantation and haploidentical HSCT[24-26]. However, there are no reported differences in engraftment between AML and ALL using one style of HSCT, especially single-unit CBT. In the present study, AML and ALL also showed no difference in engraftment for CBT, without grouping patients by age. However, in the adolescents and young adults group, the recovery of neutrophils and platelets in patients with AML was better than in those with ALL. Among adolescents and young adults, patients with ALL underwent conditioning with total body irradiation more than in patients with AML. This result was in accordance with previous findings that total body irradiation causes great injury to bone marrow stromal cells and has a high risk of infection[27]. Their results were also according to the influence of total body irradiation for injury to bone marrow stromal cells. A study indicated that regulatory T-cells play a critical role in preventing autoimmune-mediated thrombocytopenia; in addition, delayed immune reconstitution, including regulatory T-cells, may contribute to the delay of platelet recovery[28-29]. Our data of immune reconstitution also showed that Treg cells were lower in ALL than in AML. Studies in adult patients with AML and ALL have showed similar results for aGVHD (grade III-IV or grade II-IV) and cGVHD after CBT[30-32]. For pediatric AML and ALL, a few studies have also reported similar outcomes of aGVHD and cGVHD after CBT[33-34]. Our data confirmed similar outcomes of aGVHD and cGVHD between AML and ALL in both adult and pediatric patients.
Similar to findings regarding aGVHD and cGVHD between AML and ALL in previous studies, relapse in these diseases is also similar after CBT[1, 3, 21]. In age-restricted studies among adults and children, relapse in patients aged > 50 and < 1 years was similar between AML and ALL[9]. However, according to our data, relapse for ALL at 2 years was higher than AML in patients without grouping by age. In line with a previous report, our data also showed that there were no differences in relapse between AML and ALL in children. To further explore the higher relapse rate in ALL, we analyzed immune reconstitution after CBT. Our data suggested that the CD8+ T cell dose in AML for the age group 15-39 years was higher than that in ALL at 4 months after CBT. One study mentioned that early CD8+ recovery was associated with low risk of relapse and more severe GVHD[35]. In this study, the CD8+ cell count was higher in patients with ALL, who had lower relapse rates. CD8+ T cells are also considered to be related to aGVHD; in the present study, we believe that the double negative T cell levels might be related to cGVHD[36-37]. In continuous monitoring, we detected that there was no difference between AML and ALL with respect to immune reconstitution, after 1 year. Therefore, patients with AML or ALL both had decreased relapse with time.
For transplant recipients, adolescents and young adults are a special group of patients, as the disease occurs early in life, in contrast to older adults, resulting in a high burden of HSCT sequelae[38]. Earlier studies indicated that between adolescents and young adults and pediatric patients, inferior outcomes were observed with older age[39-40]. Our research showed similar results in non-relapse mortality between adolescents and young adults and pediatric patients with AML. According to our analysis, only HLA compatibility showed this difference of non-relapse mortality. Adolescents and young adults are characterized by plenty of maturing, hormonal, and psychosocial challenges, as well as developmental processes[41]. Thus, we believe that nearly all components of meaningful life experiences, which were not included in our research, are affected and are sensitive with respect to outcomes of HSCT.
This study has some limitations. First, this was a retrospective study with inherent biases and a lack of randomization. Second, the sample size was relatively small with grouping by age and weight, especially in older patients. To avoid this limitation, we did not analyze some outcomes in older groups. Therefore, additional studies are necessary to confirm the outcomes of CBT for older patients with AML and ALL.
In summary, we report the outcomes of AML and ALL after CBT using myeloablative conditioning without antithymocyte globulin, according to age groups. The patients with AML showed better outcomes of neutrophil and platelet recovery and relapse than adolescents and young adults in ALL. However, there were no differences between AML and ALL in outcomes after CBT with or without grouping by age, especially with respect to overall survival and GRFS. On the basis of this study, adults with ALL have outcomes of CBT that are as good as those of patients with AML. Therefore, CBT could be a decent choice for adults who do not have a suitable donor.
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
BACKGROUND: Umbilical cord blood hematopoietic stem cell transplantation is more and more widely used as a radical treatment for acute leukemia, but its therapeutic effect in different leukemias has not been compared. By comparing the efficacy of diseases, it can guide different patients to choose the transplantation method.
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程#br#
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||