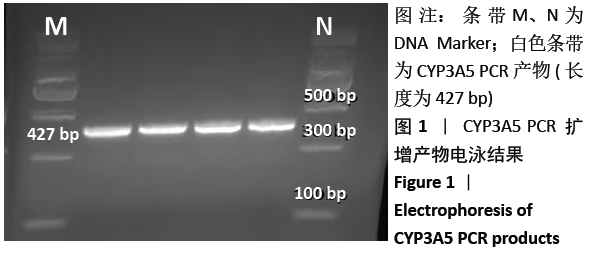
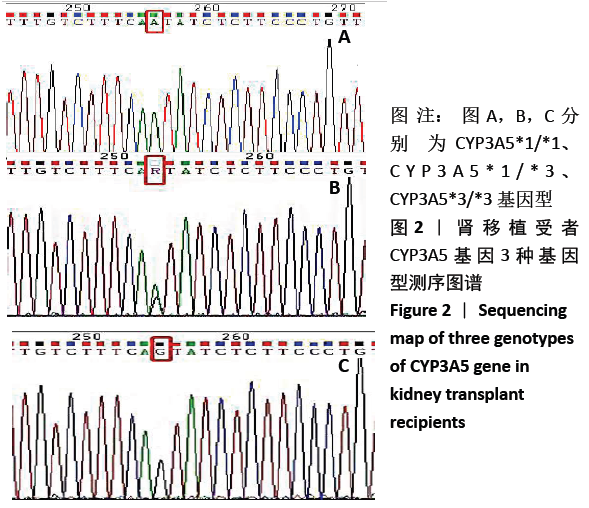
中国组织工程研究 ›› 2021, Vol. 25 ›› Issue (11): 1740-1744.doi: 10.3969/j.issn.2095-4344.3037
• 组织构建临床实践 clinical practice in tissue construction • 上一篇 下一篇
CYP3A5基因多态性与肾移植受者他克莫司浓度/剂量的关系及个体化用药
刘君昌1,2,高小林2,姜泰茂2
- 1锦州医科大学研究生院,辽宁省锦州市 121001;2中国人民解放军北部战区空军医院泌尿外科,辽宁省沈阳市 110042
Correlation of CY3A5 genetic polymorphism with concentration/dosage of tacrolimus and individualized administration of tacrolimus after kidney transplantation
Liu Junchang1, 2, Gao Xiaolin2, Jiang Taimao2
- 1Graduate School of Jinzhou Medical University, Jinzhou 121001, Liaoning Province, China; 2Department of Urology, Air Force Hospital of Northern Theater Command, Shenyang 110042, Liaoning Province, China
摘要:
文题释义:
CYP3A5:细胞色素P450酶3A5,即细胞色素P450基因,第3家族,A亚家族,第5多肽链。
他克莫司浓度/剂量(C0/D):C0 为他克莫司谷浓度 (μg/ L);D为患者每日每千克体质量所服用他克莫司毫克数[mg/(kg•d)]。
背景:CYP3A5基因多态性可显著影响肾移植术后早期他克莫司血药浓度,国内许多的研究局限于肾移植后早期3个月内,缺乏对受者术后稳定期的影响。
目的:探讨CYP3A5基因多态性与肾移植受者他克莫司浓度/剂量(C0/D)之间的关系,比较不同基因型之间的差异,为肾移植受者术后他克莫司个体化用药提供方案。
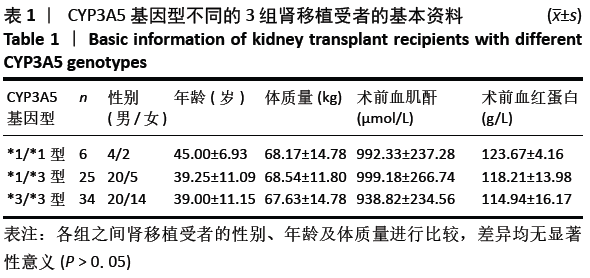
方法:接受肾移植及术后均采用他克莫司( FK506) + 霉酚酸酯(MMF) + 泼尼松(Pred)免疫抑制治疗方案的65例成人受者,术前均检测受者CYP3A5基因型,按CYP3A5*1/*1、*1/*3、*3/*3型分为3组,监测受者术后他克莫司全血谷浓度C0指标,记录不同时间点3组受者他克莫司血药C0/D值。研究的治疗方案的实施符合北部战区空军医院的相关伦理要求。
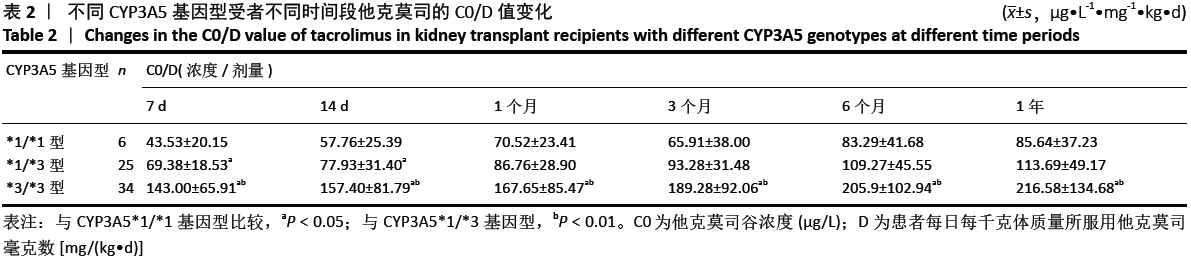
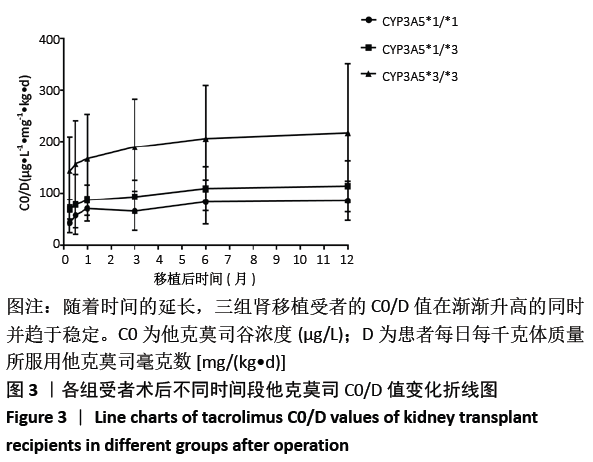
结果与结论:①CYP3A5 *1/*1、*1/*3、*3/*3基因型的肾移植受者分别是6,25和34例;②CYP3A5*1/*1和*1/*3基因型受者组术后各个时间段他克莫司C0/D比值均明显低于*3/*3基因型受者组(P < 0.05);*1/*1型受者组术后7,14 d时的他克莫司C0/D比值低于*1/*3型受者组(P=0.028,P=0.034);③*1/*1型受者组术后7d他克莫司C0/D值明显低于术后6个月、1年(P=0.35,P=0.41);*1/*3型受者组术后7 d时他克莫司C0/D值明显低于术后3,6个月及1年(P=0.029,P=0.07),术后14 d、1个月低于术后6个月、1年(P=0.04,P=0.39);*3/*3型受者术后7 d时他克莫司C0/D值明显低于术后3,6个月、1年(P=0.029,P=0.03),术后14 d明显低于术后6月、1年(P=0.022);④结果说明,CYP3A5基因多态性对肾移植受者术后的他克莫司 C0/D值有显著影响,且可维持到移植术后长时间的稳定期。CYP3A5*1/*1、*1/*3型受者早期他克莫司代谢较快,要加大他克莫司给药剂量来维持目标血药浓度;*1/*3型受者剂量可适当比前者略少,后期应减慢减药速度;*3/*3型受者他克莫司代谢缓慢,早期应给予小剂量,后期应加快减药速度。
https://orcid.org/0000-0003-3557-3469 (刘君昌)
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
中图分类号: