中国组织工程研究 ›› 2016, Vol. 20 ›› Issue (3): 418-422.doi: 10.3969/j.issn.2095-4344.2016.03.021
• 生物材料研究方案 biomaterial study protocols • 上一篇 下一篇
透明质酸钠羧甲基纤维素预防术后腹膜粘连的最佳剂量筛选及机制研究:随机对照实验方案
吴志民1, 2,崔宏力2,刘国辉3
- 1北京大学肿瘤医院暨北京市肿瘤防治研究所胃肠肿瘤中心,恶性肿瘤发病机制及转化研究教育部重点实验室,北京市 100142;2北京市垂杨柳医院普外科,北京市 100022;3吉林大学第一医院急诊外科,吉林省长春市 130021
Optimal dose and mechanism of sodium hyaluronate/carboxymethylcellulose for prevention of postoperative peritoneal adhesions: study protocol for a randomized controlled trial
Wu Zhi-min1, 2, Cui Hong-li2 , Liu Guo-hui3
- 1Center for Gastrointestinal Cancer, Beijing Cancer Hospital, Beijing Institute for Cancer Research, Beijing 100142, China; 2Department of General Surgery, Beijing Chuiyangliu Hospital, Beijing 100022, China;3Department of Emergency Surgery, the First Hospital of Jilin University, Changchun 130021, Jilin Province, China
摘要:
文章快速阅读:
.jpg)
文题释义:
腹膜粘连:是腹膜受到手术、创伤、感染、缺血等损伤后,机体在自然修复过程中必然出现的一种反应,腹膜粘连可能导致粘连性肠梗阻、慢性腹痛腹胀和女性不育等并发症发生。
透明质酸钠羧甲基纤维素:是一种胶液状高分子防粘连材料,具有不溶血、无致突变性、可降解等优势,是一种组织相容性良好的体内植入生物材料。因其具有类似体液如滑膜液的黏滞性,在上皮细胞再生期间可起到“漂浮浴”作用,阻隔浆膜面与腹膜面接触,从而防止粘连的形成。
背景:研究发现局部应用屏障物是一种行之有效的预防术后腹膜粘连的方法。前期实验已经制备了有专利的防粘连材料——透明质酸钠羧甲基纤维素,这种胶液状高分子材料生物相容性好,因其具有流动性和可压性,能够随着器官的蠕动而漫布于整个腹腔,起润滑作用,将肠管和腹膜隔开,减少组织粘连。
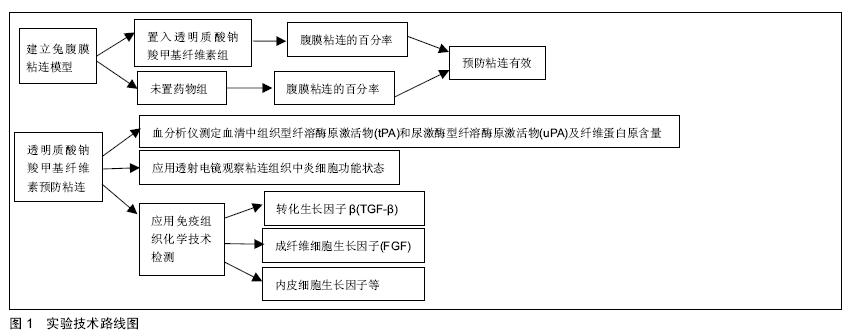
方法/设计:随机对照动物实验。①建立新西兰兔腹膜粘连模型,创面置入不同浓度及不同剂量的透明质酸钠羧甲基纤维素,与未置药物组比较腹膜粘连百分率,筛选透明质酸钠羧甲基纤维素抗腹膜粘连的最佳浓度及剂量。②透明质酸钠羧甲基纤维素抗粘连的机制研究,检测成纤维细胞生长因子、内皮细胞生长因子、转化生长因子β及血清中组织型纤溶酶原激活物(tPA)、尿激酶型纤溶酶原激活物(uPA) 和纤维蛋白原。
讨论:实验结果将为临床应用透明质酸钠羧甲基纤维素预防腹部手术后腹膜粘连提供实验依据,也为透明质酸钠羧甲基纤维素抗粘连机制探索提供可行思路。
伦理批准:研究经吉林大学第一医院伦理委员会批准,实验过程中对动物的处置符合2006年科技部《关于善待实验动物的指导性意见》及2009年《Ethical issues in animal experimentation》相关动物伦理学标准。
ORCID: 0000-0002-6691-4492(刘国辉)