3.1 lncRNAs的来源及功能特点
lncRNAs是指大于
200 nt的RNA
s,位于细胞核或胞浆内,不参与或很少参与蛋白质的编码
[12]。学者们曾经认为其是转录的“暗区”,但最新的研究已表明这些转录产物可能直接参与细胞的调控
[13],在功能上可分为4大类或原型:信号,诱饵,向导和支架在多种生物学过程中发挥作用
[14],尤其是癌症的发病过程
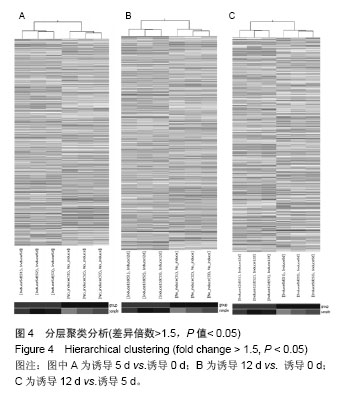
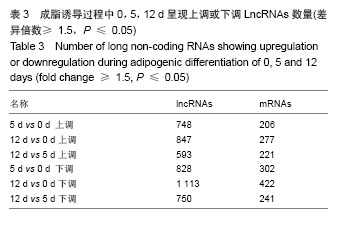
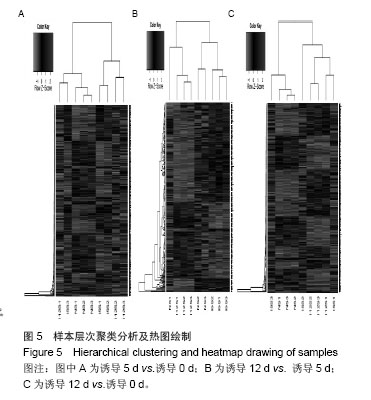
[15]。然而对于lncRNAs在人脂肪干细胞脂向分化过程中差异性表达的研究在还处于初级阶段,作者结合芯片技术及生物信息学分析,力求在脂向分化过程中发现lncRNAs的差异表达,为肥胖的治疗提供新的思路。 3.2 lncRNAs微阵列芯片(Microarrays)及生物信息学分析 作者体外培养3例人脂肪干细胞并成功脂向诱导分化,分别对诱导0,5,12 d细胞的lncRNAs运用cDNA微阵列进行芯片测序,获得数以万计的lncRNAs、mRNAs表达谱及其差异表达谱。按照差异倍数≥1.5(
P < 0.05,差异有显著性意义)进行初步筛选,不再赘述。为缩小研究范围,作者按照Fold Change≥2.0(
P < 0.01)从大量差异表达的lncRNA中进一步筛选,根据生物信息学报告从筛选结果中再进一步选择可能与脂类代谢相关的lncRNA,共28个。
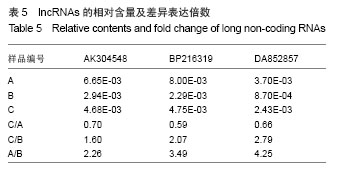
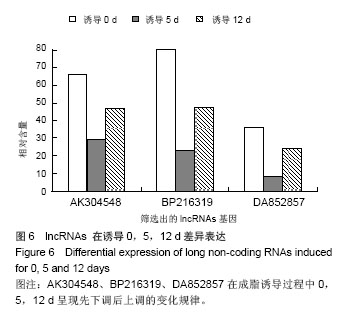
3.3 筛选目的lncRNAs 对得到的28个可能与脂类代谢相关的lncRNAs进行逐一分析,lncRNA-AK304548及其靶基因ARHGEF2在3个标本中均呈现先下调后上调的趋势,且差异倍数明显;lncRNA-BP216319及其相关的靶基因脂肪酸结合蛋白(FABP3)在3个标本中均呈现一致的先下调后上调的变化趋势,且已有大量的文献表明FABP3与脂肪代谢密切相关;lncRNA-DA852857仅在标本2、3中呈现一致的先上调后下调的趋势,且其靶基因CALD1可能由于表达量少,信号强度较低被过滤掉而导致芯片数据缺失,虽在标本1中未呈现此变化趋势,但考虑到数据质量检测,根据样本相关性及层次聚类发现标本1数据不同于标本2和3的数据,在单个基因位点数据偏差大,故仍将其列入进一步验证的lncRNA;对筛选出以上3个lncRNAs做进一步验证。
3.4 对目的lncRNAs及其可能的靶基因进qRT-PCR验证 qRT-PCR验证得出3个筛选出的lncRNAs及其对应的靶基因在0,5,12 d均呈现先下调后上调的变化趋势,这与lncRNA-AK304548及靶基因ARHGEF2,lncRNA- DA852857及靶基因FABP3芯片数据结果相符,与lncRNA-DA852857芯片数据结果不符,故说明芯片在DA852857点出现假阳性且其靶基因CALD1在芯片数据缺失,所以无法比较;因此进一步筛选出lncRNA -AK304548、BP216319及对应的靶基因ARHGEF2、FABP3留作后续研究分析。
3.5 差异表达的lncRNAs及其可能的靶基因简要分析
3.5.1 AK304548和靶基因鸟嘌呤核苷酸交换因子(ARHGEF2) ARHGEF2是鸟嘌呤核苷酸交换因子家族的一员,目前国内外并未发现对ARHGEF2功能研究的文献,所以根据ARHGEF家族基因预测其可能的作用。ARHGEF家族主要参与核内有丝分裂、微管肌动蛋白、细胞骨架等细胞增殖相关的生物学过程。Finucane等
[16]证明参与乳汁合成基因表达发生上调的同时,与细胞增殖相关的基因则受到抑制。上调表达的基因主要与转运活动(氨基酸、葡萄糖、离子的转运等)、脂类和糖代谢以及细胞信号因子相关,下调表达的基因主要与细胞周期和增殖、DNA复制和微型染色体组建、以微管为基础的过程以及蛋白质和RNA降解相关。
在实验中脂肪干细胞诱导分化0 d,细胞仍处于增殖状态,所以与细胞增殖相关的基因ARHGEF2高表达,诱导开始至5 d,细胞处于向脂肪细胞分化状态,与脂类代谢、调节转录等相关的基因及细胞因子高表达,而与增殖相关的ARHGEF2受到抑制则处于低表达状态。诱导至12 d,约80%的脂肪细胞分化完成,由于脂肪细胞的体积较分化前要大,参与细胞微管、肌动蛋白、细胞骨架等相关的基因ARHGEF2明显上调,甚至高于诱导分化前。
AK304548与其靶基因ARHGEF2呈现相同的变化趋势,这说明AK304548与ARHGEF2正向相关。AK304548位于1号染色体,是一个来源于lincRNAs的lincRNA。序列名称为HMlincRNA1024。作为信号或诱饵的lncRNAs的功能主要是参与基因的表达和调控,而作为向导和支架的lncRNAs主要是参与表观遗传修饰
[17]。根据ARHGEF2在脂肪干细胞成脂分化中受到抑制,可以推断AK304548在调控网络中可能作为信号或者诱饵发挥调节基因激活或抑制的作用。而一个lncRNA分子可能具有不同功能类型的几个功能,也可能存在复杂的功能类型或者是不同分子机制的组合
[18]。所以AK304548在脂肪干细胞脂向分化中的作用及其与ARHGEF2的调节关系需要在下一步对AK304548通过功能缺失实验,构建慢病毒载体、转染脂肪干细胞中进一步研究。
3.5.2 BP216319及其可能的靶基因脂肪酸结合蛋白(FABP3) FABP3是有效的棕色脂肪组织中的脂肪酸氧化和抗寒性的关键
[19],参与适应性发热需求
[20],在胰岛细胞中与FABP5一起由脂肪酸和葡萄糖调控
[21],在脂类代谢过程中主要参与细胞内脂肪酸转运。其代谢水平与细胞内转运及脂类代谢密切相关
[22]。脂肪细胞形成是由转录级联驱动管理的,在很大程度上依赖于过氧化物酶体增殖物激活受体γ(PPARγ),它是脂肪细胞富含的核内受体。PPARγ是脂肪形成所必须的,至今还没有发现在PPARγ不存在的情况下其他因子能够诱导脂肪形成
[10]。而FABP可能作为PPARγ表达的触发器发挥作用,但是这具体的机制还需要进一步研究
[23]。实验中在脂肪干细胞诱导分化的0 d,脂肪细胞内的脂肪酸运输处于平衡状态,当诱导开始后至5 d,脂肪细胞分化的转录调节开始活跃,PPARγ表达增加,这时脂肪酸的合成也开始活跃,由于脂肪酸合成较少,参与运输的的FABP3的表达下降,脂质堆积较少,而到诱导后12 d,近80%的脂肪细胞分化完成,一方面PPARγ的增加上调了FABP,另一方面脂质合成增多,脂肪酸运输活跃,FABP增加,脂质堆积增多。BP216319以FABP3作为靶基因,呈现与FABP3相同的变化趋势,可能在调控FABP3合成中起到正向调控作用。成脂分化开始后BP216319处于低表达水平,分化基本完成时恢复到高表达水平。这种变化趋势表明,在脂肪干细胞脂向分化时,BP216319受到抑制。如前所述,作为信号或诱饵的lncRNAs的功能主要参与基因的表达和调控,而作为向导和支架的lncRNAs主要参与表观遗传修饰
[24]。这表明BP216319可能也是作为信号或诱饵发挥调控作用。
3.6 实验中存在的局限性 本实验的局限性在于仅选取3例样本,数量较少,存在偶然性。其中标本1与标本2、3细胞的培养及芯片测序是分次进行的,可能存在误差。芯片cDNA微阵列分析lncRNAs存在假阳性及假阴性,需要一种精确度更高的技术手段来检测lncRNAs。随着对lncRNAs研究的深入,越来越多的lncRNAs被发现。现在本实验应用的芯片功能注释的lncRNAs数量较少,仍有许多刚发现的lncRNAs未纳入。鉴于国内外研究的现状,脂肪干细胞脂向分化相关的基因调控网络仍未完善,所以研究所涉及到的mRNA可能不全,有待进一步深入研究。
3.7 下一步的研究方向 增加样本量并进行芯片扫描测序,进一步选择以成脂相关基因为靶基因且差异倍数较大的lncRNAs进行qRT-PCR验证,筛选出符合芯片测序结果的lncRNAs,连同现阶段筛选出的2个lncRNAs一起分别进行过表达及功能缺失实验,构建慢病毒载体转染脂肪干细胞,并进行油红O染色鉴定,qRT-PCR检测成脂相关转录因子及成脂标记基因的表达量,Western blot定量分析成脂相关蛋白表达量,最后荧光素酶法验证其与靶基因的关系及作用。
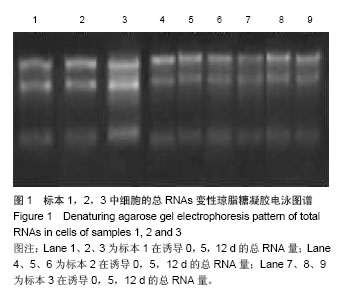
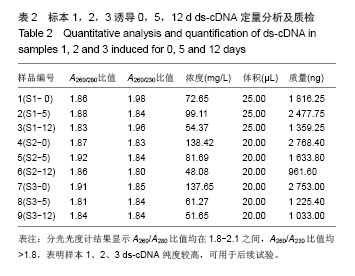
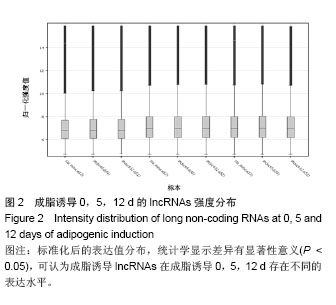
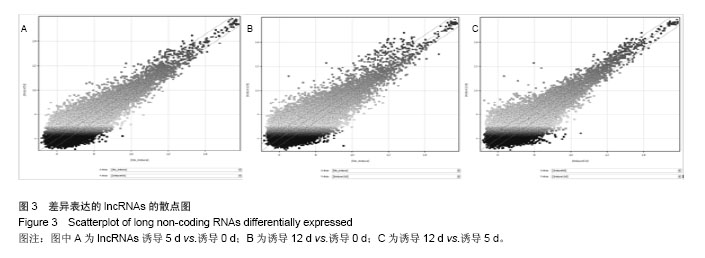

.jpg)
.jpg)