| [1] Fan WL, Liu P, Wang G, et al. Transplantation of hypoxic preconditioned neural stem cells benefits functional recovery via enhancing neurotrophic secretion after spinal cord injury in rats. J Cell Biochem. 2017 Sep 8. doi: 10.1002/jcb.26397. [Epub ahead of print][2] Suzuki H, Ahuja CS, Salewski RP, et al. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12(8):e0182339.[3] Ao Q, Wang AJ, Chen GQ, et al. Combined transplantation of neural stem cells and olfactory ensheathing cells for the repair of spinal cord injuries. Med Hypotheses. 2007;69(6):1234-1237.[4] Stewart AN, Kendziorski G, Deak ZM, et al. Co-transplantation of mesenchymal and neural stem cells and overexpressing stromal-derived factor-1 for treating spinal cord injury. Brain Res. 2017; 1672:91-105.[5] He X, Li L, Tang M, et al. Biomimetic electrical stimulation induces rat bone marrow mesenchymal stem cells to differentiate into cardiomyocyte-like cells via TGF-beta 1 in vitro. Prog Biophys Mol Biol. 2017 Sep 29. doi: 10.1016/j.pbiomolbio.2017.09.023. [Epub ahead of print][6] Guo S, Zhen Y, Wang A. Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatr Dis Treat. 2017;13: 2757-2765.[7] Cai S, Tsui YP, Tam KW, et al. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Reports. 2017;9(4):1097-1108.[8] Zhang H, Wang L, Wen S, et al. Magnetic resonance imaging tracking and assessing repair function of the bone marrow mesenchymal stem cells transplantation in a rat model of spinal cord injury. Oncotarget. 2017;8(35):58985-58999.[9] Pu Y, Meng K, Gu C, et al. Thrombospondin-1 modified bone marrow mesenchymal stem cells (BMSCs) promote neurite outgrowth and functional recovery in rats with spinal cord injury. Oncotarget. 2017; 8(56):96276-96289.[10] 莫翠萍,王家传,周光前,等.骨髓间充质干细胞移植治疗大鼠脊髓损伤的神经分化研究[J].中华细胞与干细胞杂志:电子版, 2015,5(4):33-39.[11] Ye Y, Feng TT, Peng YR, et al. The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci Lett. 2018;666:85-91. [12] 司翠平,卞合涛,闫中瑞,等.Wnt信号在骨髓间充质干细胞神经分化中的研究进展[J].中华脑科疾病与康复杂志:电子版, 2015,5(3):192-195.[13] Zhang W, Zeng YS, Zhang XB, et al. Combination of adenoviral vector-mediated neurotrophin-3 gene transfer and retinoic acid promotes adult bone marrow cells to differentiate into neuronal phenotypes. Neurosci Lett. 2006;408(2):98-103.[14] Polisetti N, Chaitanya VG, Babu PP, et al. Isolation, characterization and differentiation potential of rat bone marrow stromal cells. Neurol India. 2010;58(2):201-208.[15] Zhang W, Zhang F, Shi H, et al. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS One. 2014;9(2):e88794.[16] Zhu X, Du J, Liu G. The comparison of multilineage differentiation of bone marrow and adipose-derived mesenchymal stem cells. Clin Lab. 2012;58(9-10):897-903.[17] Song K, Huang M, Shi Q, et al. Cultivation and identification of rat bone marrow-derived mesenchymal stem cells. Mol Med Rep. 2014;10(2): 755-760.[18] Niu CC, Lin SS, Yuan LJ, et al. Identification of mesenchymal stem cells and osteogenic factors in bone marrow aspirate and peripheral blood for spinal fusion by flow cytometry and proteomic analysis. J Orthop Surg Res. 2014;9:32.[19] Zhang HT, Liu ZL, Yao XQ, et al. Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy. 2012;14(10):1203-1214.[20] Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199-2204.[21] Han S, Wang B, Li X, et al. Bone marrow-derived mesenchymal stem cells in three-dimensional culture promote neuronal regeneration by neurotrophic protection and immunomodulation. J Biomed Mater Res A. 2016;104(7):1759-1769.[22] Feng L, Gan H, Zhao W, et al. Effect of transplantation of olfactory ensheathing cell conditioned medium induced bone marrow stromal cells on rats with spinal cord injury. Mol Med Rep. 2017;16(2): 1661-1668.[23] Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181-196.[24] Lin RZ, Melero-Martin JM. Fibroblast growth factor-2 facilitates rapid anastomosis formation between bioengineered human vascular networks and living vasculature. Methods. 2012;56(3):440-451.[25] Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res. 2006;312(5):527-537.[26] Kim HM, Hwang DH, Lee JE, et al. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009;4(3): e4987.[27] Ng MT, Stammers AT, Kwon BK. Vascular disruption and the role of angiogenic proteins after spinal cord injury. Transl Stroke Res. 2011; 2(4):474-491.[28] Herrera JJ, Sundberg LM, Zentilin L, et al. Sustained expression of vascular endothelial growth factor and angiopoietin-1 improves blood-spinal cord barrier integrity and functional recovery after spinal cord injury. J Neurotrauma. 2010;27(11):2067-2076.[29] Bai Y, Yin G, Huang Z, et al. Localized delivery of growth factors for angiogenesis and bone formation in tissue engineering. Int Immunopharmacol. 2013;16(2):214-223.[30] Zhang YJ, Zhang W, Lin CG, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2012; 313(1-2):64-74. |
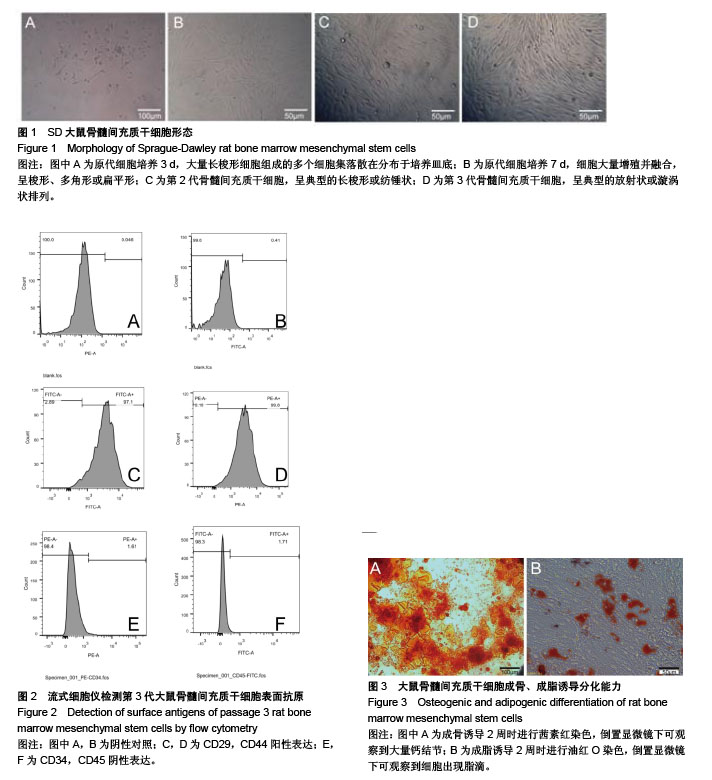
.jpg)
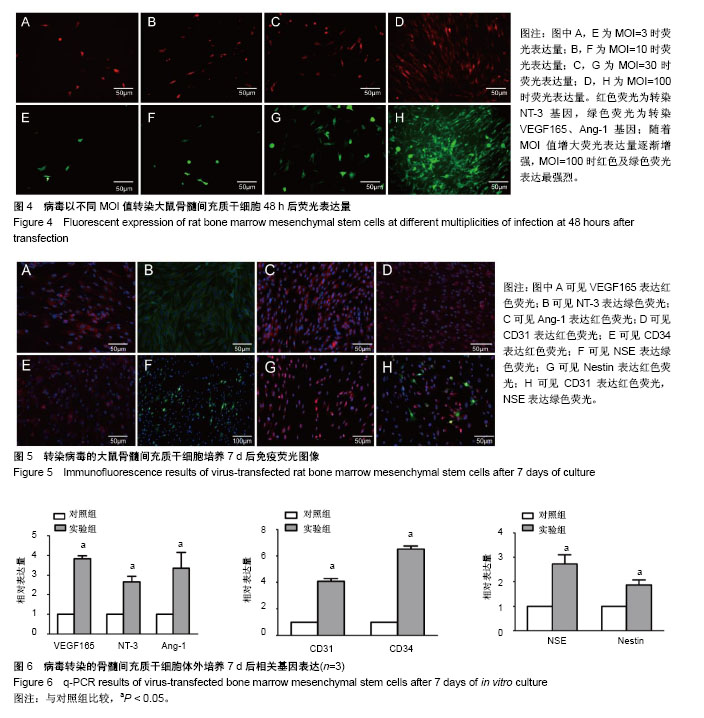
.jpg)
.jpg)