| [1] Rettig JR, Folch A. Large-scale single-cell trapping and imaging using microwell arrays. Anal Chem. 2005;77(17): 5628-5634.[2] Bratten CD, Cobbold PH, Cooper JM. Single-cell measurements of purine release using a micromachined electroanalytical sensor. Anal Chem. 1998;70(6):1164-1170.[3] Inoue I, Wakamoto Y, Moriguchi H, et al. On-chip culture system for observation of isolated individual cells. Lab Chip. 2001;1(1):50-55.[4] Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14(6):357-368.[5] Clevers H.The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313-319.[6] Altschuler SJ, Wu LF. Cellular heterogeneity: do differences make a difference. Cell. 2010;141(4):559-563.[7] Di Carlo D, Tse HTK, Gossett DR. Introduction: Why Analyze Single Cells? Clifton:Humana Press. 2012. [8] Ulloa-Aguirre A, Conn PM. Cellular Endocrinology in Health and Disease. Elsevier, 2014.[9] Clark P, Dunn GA, Knibbs A, et al. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int J Biochem Cell Biol. 2002;34(7):816-825.[10] Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008; 105(36):13427-13432.[11] Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14(9): 618-630.[12] Dertinger SKW, Chiu DT, And NLJ, et al. Generation of Gradients Having Complex Shapes Using Microfluidic Networks. Analytical Chemistry. 2001; 73(6):1240-1246.[13] Tanyeri M, Johnson-Chavarria EM, Schroeder CM. Hydrodynamic trap for single particles and cells. Appl Phys Lett. 2010;96(22):224101.[14] Tanyeri M, Ranka M, Sittipolkul N, et al. A microfluidic-based hydrodynamic trap: design and implementation Lab Chip. 2011;11(10):1786-1794.[15] Yu M, Chen Z, Xiang C, et al. Microfluidic-based single cell trapping using a combination of stagnation point flow and physical barrier. Acta Mechanica Sinica. 2016; 32(3):422-429.[16] Toriello NM, Douglas ES, Mathies RA. Microfluidic device for electric field-driven single-cell capture and activation. Anal Chem. 2005;77(21):6935-6941.[17] Arakawa T, Noguchi M, Sumitomo K, et al. High-throughput single-cell manipulation system for a large number of target cells. Biomicrofluidics. 2011;5(1):14114.[18] Jin D, Deng B, Li JX, et al. A microfluidic device enabling high-efficiency single cell trapping. Biomicrofluidics. 2015; 9(1):014101.[19] Kemna EW, Schoeman RM, Wolbers F, et al. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip. 2012;12(16): 2881-2887.[20] He M, Edgar JS, Jeffries GD, et al. Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal Chem. 2005;77(6): 1539-1544.[21] Schoendube J, Wright D, Zengerle R, et al. Single-cell printing based on impedance detection. Biomicrofluidics. 2015;9(1):014117.[22] Chabert M, Viovy JL. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc Natl Acad Sci U S A. 2008;105(9):3191-3196.[23] Kamalakshakurup G, Lee AP. High-efficiency single cell encapsulation and size selective capture of cells in picoliter droplets based on hydrodynamic micro-vortices. Lab Chip. 2017;17(24):4324-4333.[24] Ye F, Jiang J, Chang H, et al. Improved single-cell culture achieved using micromolding in capillaries technology coupled with poly (HEMA). Biomicrofluidics. 2015;9(4): 044106.[25] Ye F, Ma B, Gao J, et al. Fabrication of polyHEMA grids by micromolding in capillaries for cell patterning and single-cell arrays. J Biomed Mater Res B Appl Biomater. 2015;103(7): 1375-1380.[26] Liu X, Liu Y, Zhao F, et al. Regulation of cell arrangement using a novel composite micropattern. J Biomed Mater Res A. 2017;105(11):3093-3101.[27] Khademhosseini A, Yeh J, Jon S, et al. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4(5):425-430.[28] Park JY, Morgan M, Sachs AN, et al. Single cell trapping in larger microwells capable of supporting cell spreading and proliferation. Microfluid Nanofluidics. 2010;8(2):263-268.[29] Kim SH, Yamamoto T, Fourmy D, et al. Electroactive microwell arrays for highly efficient single-cell trapping and analysis. Small. 2011;7(22):3239-3247.[30] Zheng S, Lin H, Liu JQ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162(2): 154-161.[31] Lindström S, Eriksson M, Vazin T, et al. High-density microwell chip for culture and analysis of stem cells. PLoS One. 2009;4(9):e6997.[32] Lecault V, Vaninsberghe M, Sekulovic S, et al. High-throughput analysis of single hematopoietic stem cell proliferation in microfluidic cell culture arrays. Nat Methods. 2011;8(7):581-586.[33] Choi J, Bae S, Kim K, et al. Capture and culturing of single microalgae cells, and retrieval of colonies using a perforated hemispherical microwell structure. Rsc Advances.2014; 4(106):61298-61304.[34] Charnley M, Textor M, Khademhosseini A, et al. Integration column: microwell arrays for mammalian cell culture. Integr Biol (Camb). 2009;1(11-12):625-634.[35] Lin CH, Hsiao YH, Chang HC, et al. A microfluidic dual-well device for high-throughput single-cell capture and culture. Lab Chip. 2015;15(14):2928-2938. |
.jpg)
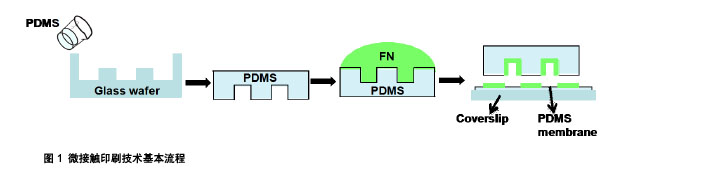
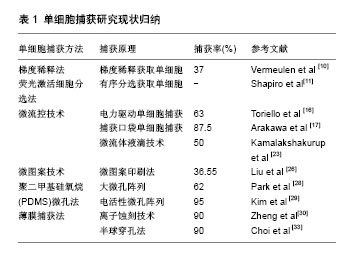
.jpg)