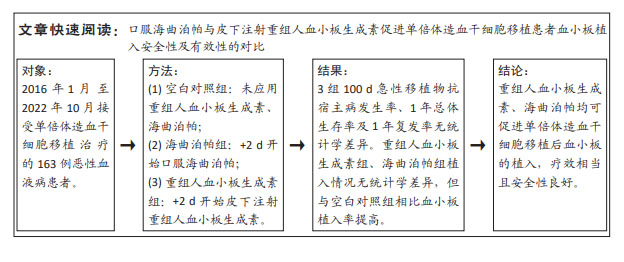
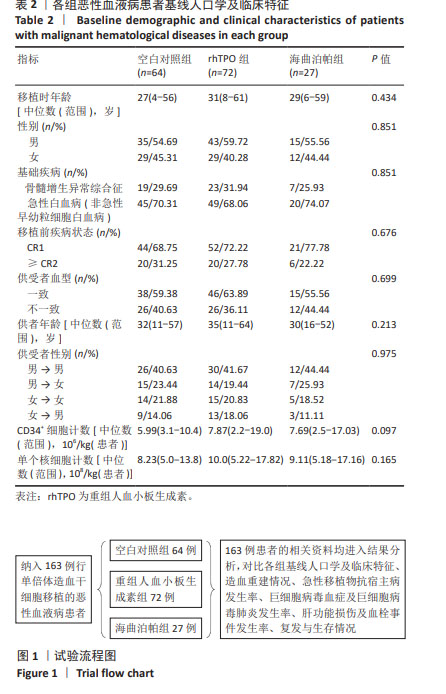
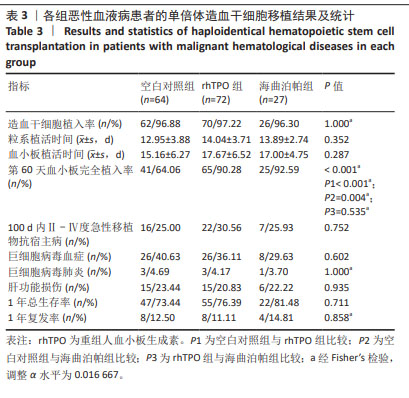
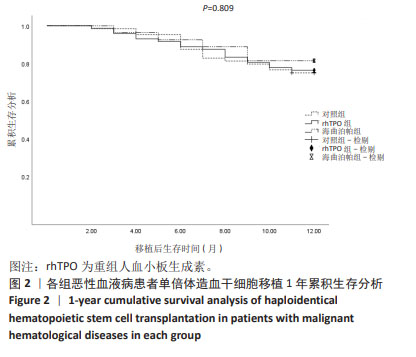
[1] YAMAZAKI R, KUWANA M, MORI T, et al. Prolonged thrombocytopenia
affer allogeneic hematopoiettc stem cell transplantatton: associattons
with impaired platelet productton and increased platelet turnover.
Bone Marrow Transplant. 2006;38(5):377-384.
[2] HUANG AJ, HU XX, WANG JM. Research Progress on
Thrombocytopenia affer Allogeneic Hematopoiettc Stem Cell
Transplantatton -Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017;
25(1):270-275.
[3] HUANG XJ. Current status of haploidenttcal stem cell transplantatton
for leukemia. J Hematol Oncol. 2008;1:27.
[4] KANDA Y, CHIBA S, HIRAI H, et al. Allogeneic hematopoiettc stem
cell transplantatton from family members other than HLA-identtcal
siblings over the last decade (1991-2000). Blood. 2003;102(4):
1541-1547.
[5] KUZMINA Z, EDER S, BÖHM A, et al. Signiffcantly worse survival of
pattents with NIH-deffned chronic graff-versus-host disease and
thrombocytopenia or progressive onset type: results of a prospecttve
study. Leukemia. 2012;26(4):746-756.
[6] ALEXANDER WS, ROBERTS AW, NICOLA NA, et al. Deffciencies in
progenitor cells of multtple hematopoiettc lineages and defecttve
megakaryocytopoiesis in mice lacking the thrombopoiettc receptor
c-Mpl. Blood. 1996;87(6):2162-2170.
[7] HAN TT, XU LP, LIU DH, et al. Recombinant human thrombopoiettn
promotes platelet engraffment affer haploidenttcal hematopoiettc
stem cell transplantatton: a prospecttve randomized controlled trial.
Ann Hematol. 2015;94(1):117-128.
[8] BUSSEL JB, SOFF G, BALDUZZI A, et al. A Review of Romiplosttm
Mechanism of Actton and Clinical Applicability. Drug Des Devel Ther.
2021;15:2243-2268.
[9] WEN B, ZHANG X, CHEN S, et al. Oral eltrombopag versus
subcutaneous recombinant human thrombopoiettn for promottng
platelet engraffment affer allogeneic stem cell transplantatton: A
prospecttve, non-inferiority, randomized controlled trial. Hematol
Oncol. 2022;40(4):777-786.
[10] MEI H, LIU X, LI Y, et al. A multtcenter, randomized phase III trial
of hetrombopag: a novel thrombopoiettn receptor agonist for the
treatment of immune thrombocytopenia. J Hematol Oncol. 2021;
14(1):37.
[11] ZHANG F, PENG G, HE G, et al. A Multtcenter, Open-Label, Single-Arm,
Phase 2 Study to Evaluate the Efffcacy and Safety of Hetrombopag in
Pattents with Severe Aplasttc Anemia (SAA). Blood. 2020;136:3-4.
[12] XIE C, ZHAO H, BAO X, et al. Pharmacological characterizatton of
hetrombopag, a novel orally acttve human thrombopoiettn receptor
agonist. J Cell Mol Med. 2018;22(11):5367-5377.
[13] 孔黛 , 陈香丽 , 裴晓杭 , 等 . 去除第 11 天甲氨蝶呤对单倍体造血干
细胞移植患者发生急性移植物抗宿主病的影响 [J]. 中国组织工程
研究 ,2022,26(31):5026-5031.
[14] SCHOEMANS HM, LEE SJ, FERRARA JL, et al. EBMT-NIH-CIBMTR Task
Force positton statement on standardized terminology & guidance
for graff-versus-host disease assessment. Bone Marrow Transplant.
2018;53(11):1401-1415.
[15] FORMAN SJ, NEGRIN RS, ANTIN JH, et al. Thomas’ Hematopoiettc Cell
Transplantatton: Stem Cell Transplantatton[M]. 5th Ed. West Sussex, UK:
John Wiley & Sons, Ltd. 2015:1012-1019.
[16] ALSHEMMARI S, AMEEN R, GAZIEV J. Haploidenttcal hematopoiettc
stem-cell transplantatton in adults. Bone Marrow Res. 2011;2011:
303487.
[17] BLUMBERG N, HEAL JM, PHILLIPS GL, et al. Platelets--to transfuse or
not to transfuse. Lancet. 2012;380(9850):1287-1289.
[18] ZHANG X, FU H, XU L, et al. Prolonged thrombocytopenia following
allogeneic hematopoiettc stem cell transplantatton and its associatton
with a reductton in ploidy and an immaturatton of megakaryocytes. Biol
Blood Marrow Transplant. 2011;17(2):274-280.
[19] MARSH JC, MUFTI GJ. Eltrombopag: a stem cell cookie? Blood. 2014;
123(12):1774-1775.
[20] KUTER DJ. New thrombopoiettc growth factors. Blood. 2007;109(11):
4607-4616.
[21] COHN CS, BUSSEL JB. Romiplosttm: a second-generatton
thrombopoiettn agonist. Drugs Today (Barc). 2009;45(3):175-188.
[22] KAPUR R, ASLAM R, SPECK ER, et al. Thrombopoiettn receptor agonist
(TPO-RA) treatment raises platelet counts and reduces antt-platelet
anttbody levels in mice with immune thrombocytopenia (ITP). Platelets.
2020;31(3):399-402.
[23] POLVERELLI N, CATANI L, SOLLAZZO D, et al. Platelet ffuctuattons during
thrombopoiettn-receptor agonist treatment: correlatton with platelet
apoptosis. Ann Hematol. 2015;94(2):339-341.
[24] CHINESE SOCIETY OF HEMATOLOGY, CHINESE MEDICAL ASSOCIATION.
Chinese expert consensus on the management of hemorrhagic
complicattons affer hematopoiettc stem cell transplantatton(2021).
Zhonghua Xue Ye Xue Za Zhi. 2021;42(4):276-280.
[25] AHMED S, BASHIR Q, BASSETT R, et al. Eltrombopag for PostTransplantatton
Thrombocytopenia: Results of Phase II Randomized,
Double-Blind, Placebo-Controlled Trial. Transplant Cell Ther. 2021;27(5):
430.e1-430.e7.
[26] LIESVELD JL, PHILLIPS GL 2ND, BECKER M, et al. A phase 1 trial of
eltrombopag in pattents undergoing stem cell transplantatton affer
total body irradiatton. Biol Blood Marrow Transplant. 2013;19(12):
1745-1752.
[27] CATALÁ-LÓPEZ F, CORRALES I, DE LA FUENTE-HONRUBIA C, et al.
Risk of thromboembolism with thrombopoiettn receptor agonists in
adult pattents with thrombocytopenia: Systemattc review and metaanalysis
of randomized controlled trials. Med Clin (Barc). 2015;145(12):
511-519.
[28] MITTELMAN M, PLATZBECKER U, AFANASYEV B, et al. Eltrombopag for
advanced myelodysplasttc syndromes or acute myeloid leukaemia and
severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled,
phase 2 trial. Lancet Haematol. 2018;5(1):e34-e43.
[29] PEFFAULT DE LATOUR R, KULASEKARARAJ A, IACOBELLI S, et al.
Eltrombopag Added to Immunosuppression in Severe Aplasttc Anemia.
N Engl J Med. 2022;386(1):11-23.
[30] WOLFF SN, HERZIG R, LYNCH J, et al. Recombinant human
thrombopoiettn (rhTPO) affer autologous bone marrow transplantatton:
a phase I pharmacokinettc and pharmacodynamic study. Bone Marrow
Transplant. 2001;27(3):261-268.
[31] ZHENG X, ZHANG H, GUO W, et al. Herombopag promotes
platelet engraffment and decreases platelet transfusion affer
allogeneic hematopoiettc stem cell transplantatton. Eur J Haematol.
2023;110(5):527-533.
[32] GHANIMA W, JUNKER P, HASSELBALCH HC, et al. Fibroproliferattve
acttvity in pattents with immune thrombocytopenia (ITP) treated with
thrombopoiettc agents. Br J Haematol. 2011;155(2):248-255.
[33] GHANIMA W, GEYER JT, LEE CS, et al. Bone marrow ffbrosis in 66
pattents with immune thrombocytopenia treated with thrombopoiettnreceptor
agonists: a single-center, long-term follow-up. Haematologica.
2014;99(5):937-944.
[34] MOUSTAFA I, FETOUH SM, BADAWY ME. Eltrombopag-Induced
Myeloffbrosis in Pattents with Adult Immune Thrombocytopenia:
Scoping Review. EMJ Hematol. 2019;7(1):69-79.
[35] GHANIMA W, COOPER N, RODEGHIERO F, et al. Thrombopoiettn
receptor agonists: ten years later. Haematologica. 2019;104(6):
1112-1123.
|