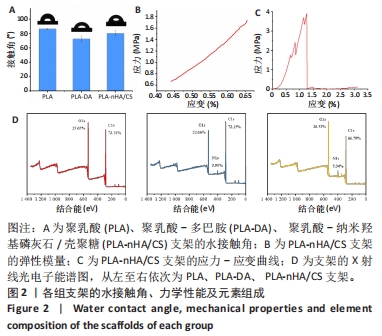
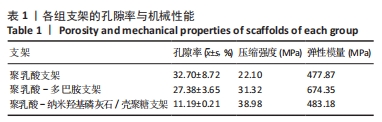
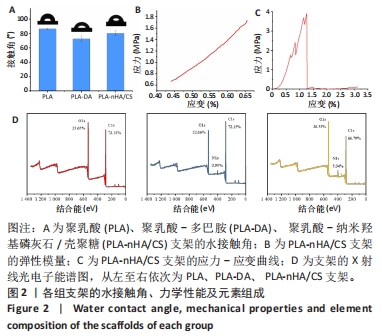
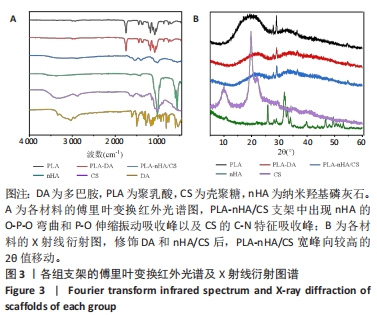
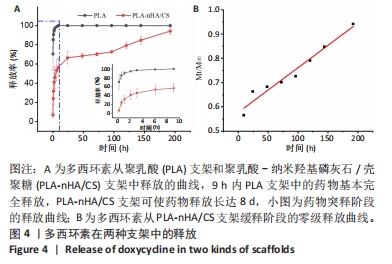
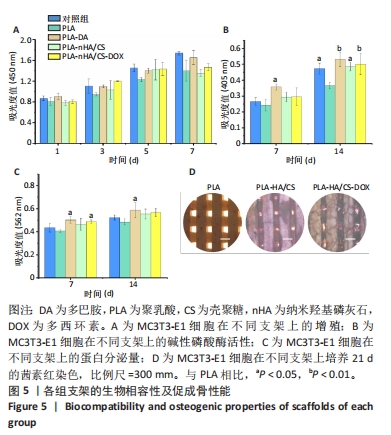
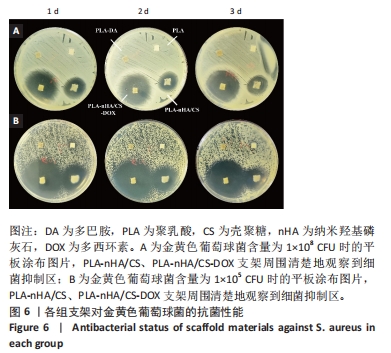
[1] WUBNEH A, TSEKOURA EK, AYRANCI C, et al. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018;80:1-30.
[2] KOONS GL, DIBA M, MIKOS AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5(8):584-603.
[3] TETIK H, FENG D, OXANDALE SW, et al. Bioinspired manufacturing of aerogels with precisely manipulated surface microstructure through controlled local temperature gradients. ACS Appl Mater Interfaces. 2021;13(1):924-931.
[4] ALDEMIR DIKICI BL, REILLY GC, CLAEYSSENS F. Boosting the osteogenic and angiogenic performance of multiscale porous polycaprolactone scaffolds by in vitro generated extracellular matrix decoration. ACS Appl Mater Interfaces. 2020;12(11):12510-12524.
[5] XING H, ZOU B, LIU X, et al. Fabrication strategy of complicated Al2O3-Si3N4 functionally graded materials by stereolithography 3D printing. J Eur Ceram Soc. 2020;40(15):5797-5809.
[6] BOULAALA M, ELMESSAOUDI D, BUJ-CORRAL I, et al. Towards design of mechanical part and electronic control of multi-material/multicolor fused deposition modeling 3D printing. Int J Adv Manuf Tech. 2020; 110:45-55.
[7] ZHANG W, FENG C, YANG G, et al. 3D-printed scaffolds with synergistic effect of hollow-pipe structure and bioactive ions for vascularized bone regeneration. Biomaterials. 2017;135:85-95.
[8] LIM SH, CHIA SMY, KANG L, et al. Three-Dimensional Printing of Carbamazepine Sustained-Release Scaffold. J Pharm Sci. 2016;105(7): 2155-2163.
[9] YANG Y, QIAO X, HUANG R, et al. E-jet 3D printed drug delivery implants to inhibit growth and metastasis of orthotopic breast cancer. Biomaterials. 2020;230:119618.
[10] GAO C, PENG S, FENG P, et al. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5(1):1-33.
[11] BAE EB, PARK KH, SHIM JH, et al. Efficacy of rhBMP-2 loaded PCL/β-TCP/bdECM scaffold fabricated by 3D printing technology on bone regeneration. Biomed Res Int. 2018;2018:1-12.
[12] TERTULIANO OA, GREER JR. The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater. 2016;15(11):1195-1202.
[13] CROSS LM, THAKUR A, JALILI NA, et al. Nanoengineered biomaterials for repair and regeneration of orthopedic tissue interfaces. Acta Biomater. 2016;42:2-17.
[14] QUAN H, HE Y, SUN J, et al. Chemical self-assembly of multifunctional hydroxyapatite with a coral-like nanostructure for osteoporotic bone reconstruction. ACS Appl Mater Interfaces. 2018;10(30):25547-25560.
[15] REZWAN K, CHEN Q, BLAKER JJ, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413-3431.
[16] GHORBANI F, ZAMANIAN A, BEHNAMGHADER A, et al. A facile method to synthesize mussel-inspired polydopamine nanospheres as an active template for in situ formation of biomimetic hydroxyapatite. Mat Sci Eng C Mater. 2019;94:729-739.
[17] LÓPEZ-IGLESIAS C, BARROS J, ARDAO I, et al. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr Polym. 2019;204:223-231.
[18] FERREIRA M, RZHEPISHEVSKA O, GRENHO L, et al. Levofloxacin-loaded bone cement delivery system: Highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int J Pharm. 2017;532(1):241-248.
[19] BAUMANN B, JUNGST T, STICHLER S, et al. Control of nanoparticle release kinetics from 3D printed hydrogel scaffolds. Angew Chem Int Ed. 2017;56(16):4623-4628.
[20] MARTIN V, RIBEIRO IA, ALVES MM, et al. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mat Sci Eng C Mater. 2019;101:15-26.
[21] HUANG Y, ZHAO X, ZHANG Z, et al. Degradable gelatin-based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem Mater. 2020;32(15):6595-6610.
[22] XIE C, LI P, LIU Y, et al. Preparation of TiO2 nanotubes/mesoporous calcium silicate composites with controllable drug release. Mat Sci Eng C-Mater. 2016;67:433-439.
[23] GOUDARZI A, SADRNEZHAAD SK, JOHARI N. The prominent role of fully-controlled surface co-modification procedure using titanium nanotubes and silk fibroin nanofibers in the performance enhancement of Ti6Al4V implants - ScienceDirect. Surf Coat Technol. 2021;412:127001.
[24] MATSUMOTO T, TASHIRO Y, KOMASA S, et al. Effects of surface modification on adsorption behavior of cell and protein on titanium surface by using quartz crystal microbalance system. Materials. 2020; 14(1):97.
[25] JIA Z, SHI Y, XIONG P, et al. From solution to biointerface: graphene self-assemblies of varying lateral sizes and surface properties for biofilm control and osteodifferentiation. ACS Appl Mater Interfaces. 2016;8(27):17151-17165.
[26] GITTENS RA, SCHEIDELER L, RUPP F, et al. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014;10(7):2907-2918.
[27] GOMES FILHO MS, OLIVEIRA FA, BARBOSA MAA. A statistical mechanical model for drug release: Investigations on size and porosity dependence. Physica A. 2016;460:29-37.
[28] WANG Y, SHI X, REN L, et al. Poly (lactide‐co‐glycolide)/titania composite microsphere‐sintered scaffolds for bone tissue engineering applications. J Biomed Mater Res B. 2010;93(1):84-92.
[29] SUN H, ZHANG C, ZHANG B, et al. 3D printed calcium phosphate scaffolds with controlled release of osteogenic drugs for bone regeneration. Chem Eng J. 2022;427:130961.
[30] OLIVEIRA JE, MATTOSO LH, ORTS WJ, et al. Structural and morphological characterization of micro and nanofibers produced by electrospinning and solution blow spinning: a comparative study. Adv Mater Sci Eng. 2013;2013:1-14.
[31] NAZEER MA, YILGOR E, YAGCI MB, et al. Effect of reaction solvent on hydroxyapatite synthesis in sol–gel process. R Soc Open Sci. 2017; 4(12):171098.
[32] WU C, ZHANG Y, ZHOU Y, et al. A comparative study of mesoporous glass/silk and non-mesoporous glass/silk scaffolds: physiochemistry and in vivo osteogenesis. Acta Biomater. 2011;7(5):2229-2236.
[33] VIMALRAJ S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene. 2020;754:144855.
[34] SHI X, LI L, OSTROVIDOV S, et al. Stretchable and micropatterned membrane for osteogenic differentation of stem cells. ACS Appl Mater Interfaces. 2014;6(15):11915-11923.
[35] YU Y, LI X, LI J, et al. Dopamine-assisted co-deposition of hydroxyapatite-functionalised nanoparticles of polydopamine on implant surfaces to promote osteogenesis in environments with high ROS levels. Mat Sci Eng C Mater. 2021;131:112473.
[36] FITZPATRICK V, MARTíN-MOLDES Z, DECK A, et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials. 2021;276:120995.
[37] CAMPANA V, MILANO G, PAGANO E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25:2445-2461.
[38] BAUER T, MUSCHLER G. Bone graft materials. An overview of the basic science. Clin Oryhop Relat R. 2000;371:10-27.
[39] INZANA JA, OLVERA D, FULLER SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026-4034.
[40] GAO C, PENG S, FENG P, et al. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5(4):17059.
[41] KRASOWSKA A, SIGLER K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:112.
[42] QUAN H, HE Y, SUN J, et al. Chemical Self-Assembly of Multifunctional Hydroxyapatite with a Coral-like Nanostructure for Osteoporotic Bone Reconstruction. ACS Appl Mater Interfaces. 2018;10(30):25547-25560.
[43] ZHANG B, WANG L, SONG P, et al. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des. 2021;201:109490.
[44] CAMPOCCIA D, MONTANARO L, ARCIOLA CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331-2339.
[45] VISSCHER L, DANG HP, KNACKSTEDT M, et al. 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics. Mat Sci Eng C Mater. 2018; 87:78-89. |