1.1 设计 材料学及医用生物材料的生物安全性评价实验。
1.2 时间及地点 材料制备和形貌成分表征实验于2021年7-10月在武汉理工大学材料复合新技术国家重点实验室完成;生物安全性评价实验委托第三方检测机构青岛科创质量检测有限公司,依据国家标准《医疗器械生物学评价》要求在2021年9月至2022年7月于山东青岛完成。
1.3 材料
1.3.1 主要试剂与材料 氯化钙(中国国药集团化学试剂有限公司);磷酸氢二钠(上海麦克林生化科技有限公司);外消旋聚乳酸(相对分子质量10万,济南岱罡生物工程有限公司);二氯甲烷(中国国药集团化学试剂有限公司);聚乙烯醇(阿拉丁试剂有限公司);MEM 完全培养基(武汉普诺赛生命科技有限公司);生理盐水(山东齐都药业有限公司);棉籽油(山东西亚化学工业有限公司);高密度聚乙烯树脂(山东优索化工科技有限公司);二乙基二硫代氨基甲酸锌(上海麦克林生物化学有限公司)。
1.3.2 主要仪器 电子天平(XB220A,Precisa);低速离心机(TD4,上海卢湘仪离心机有限公司);聚能式超声波材料乳化分散(XO,南京先欧仪器制造有限公司);恒温磁力搅拌器(HJ-3,常州国华电器有限公司);恒速电动搅拌器(JJ-1B,常州澳华仪器有限公司);磁力加热搅拌器(HJ-6,金坛区西城新瑞仪器厂),真空冷冻干燥机(LGJ-12,北京松源华兴科技发展有限公司);傅里叶红外光谱仪(Thermo Scientific Nicolet iS20,赛默飞世尔科技公司);台式恒温摇床(TS-50H,上海百典仪器设备有限公司);扫描电子显微镜(JSM-IT200型,日本电子株式会社);透射电子显微镜(JEM-2100F型,日本电子株式会所)。
1.3.3 实验动物 健康初成年新西兰兔18只,雄性,体质量2.0-3.0 kg,由济南西岭角养殖繁育有限公司提供,生产许可证号:SCXK(鲁)2020 0004。健康豚鼠40只,雄性,初成年,体质量300-400 g,由济南西岭角养殖繁育有限公司提供,生产许可证号:SCXK(鲁)2020 0004。健康KM小鼠20只,初成年,雄性,体质量32-38 g,由济南朋悦实验动物繁育有限公司提供,生产许可证号:SCXK(鲁)2019 0003。
每天供给适宜的商品动物饲料,每天给予充足的清洁饮用水,饲料和饮水中的污染预计不会对实验结果有潜在的影响,温度控制在19-25 ℃范围,相对湿度控制在30%-70%范围,光照采用12 h明12 h 暗。实验经武汉理工大学实验动物伦理委员会审议批准[WHUT动(伦)2022-040号]。实验过程遵循了国际兽医学编辑协会《关于动物伦理与福利的作者指南共识》和本地及国家法规。实验动物在麻醉下进行所有的手术,并尽一切努力最大限度地减少其疼痛、痛苦和死亡。
1.4 实验方法
1.4.1 纳米羟基磷灰石的制备 采用沉淀法合成纳米羟基磷灰石,具体制备过程如下:将氯化钙溶解于超纯水中制备
0.033 4 mol/L的氯化钙溶液,将磷酸氢二钠溶解于超纯水中制备0.02 mol/L的磷酸氢二钠溶液;将等体积的磷酸氢二钠溶液迅速加入正在搅拌的氯化钙溶液中,混合溶液中Ca/P=1.67;调控反应温度为80 ℃,pH值在9-10之间,反应时间为
1 h,反应完毕后静置24 h;将白色沉淀离心(4 000 r/min离心
5 min)沉淀4次,冷冻干燥成粉末备用。
1.4.2 聚乳酸/纳米羟基磷灰石复合微球的制备 采用乳液溶剂挥发法制备复合微球:将5 g的聚乳酸溶解于100 mL二氯甲烷溶剂中;将0.5 g的纳米羟基磷灰石加入到含聚乳酸的二氯甲烷溶液中,机械搅拌20 min后冰水浴超声15 min,得到油相。再将5 g的醇溶解于1 L超纯水中,在80 ℃下水浴锅中搅拌溶解1 h左右,停止加热并冷却至室温,得到水相。将含有纳米羟基磷灰石和聚乳酸的二氯甲烷溶液倒入聚乙烯醇溶液中,采用恒速电动搅拌器(转速为1 000 r/min)机械搅拌约11 h,固化得到聚乳酸/纳米羟基磷灰石复合微球;搅拌停止后静置过夜,将白色沉淀离心(4 000 r/min离心5 min)后冷冻干燥,得到聚乳酸/纳米羟基磷灰石复合微球粉体(纳米羟基磷灰石质量比为10%)。
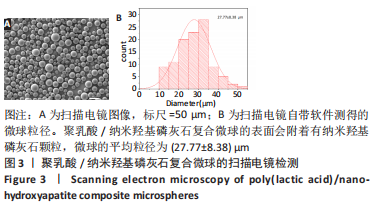
1.4.3 聚乳酸/纳米羟基磷灰石复合微球的形貌特征 将纳米羟基磷灰石处理后,用透射电子显微镜分析其外貌及形状。取聚乳酸/纳米羟基磷灰石复合微球超声分散于乙醇中,处理后用扫描电子显微镜分析其外貌及形状。
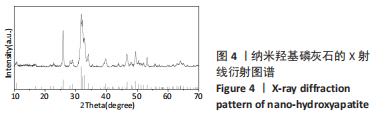
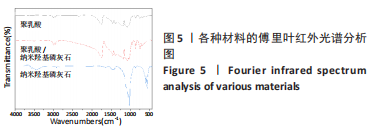
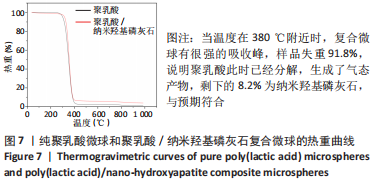
1.4.4 聚乳酸/纳米羟基磷灰石复合微球的成分分析 将纳米羟基磷灰石处理后用X射线衍射仪(D8 Advance)分析其相成分。在4 000-400 cm-1红外扫描波长范围内,对纯聚乳酸微球、聚乳酸/纳米羟基磷灰石复合微球和纳米羟基磷灰石进行傅里叶红外光谱分析。选用STA449F3型同步热分析仪对干燥后的纯聚乳酸微球和聚乳酸/纳米羟基磷灰石复合微球进行热重分析,氩气氛围下,从室温下以20 ℃/min的升温速率至1 000 ℃。取聚乳酸/纳米羟基磷灰石复合微球超声分散于乙醇中,处理后用扫描电子显微镜进行面扫描分析。
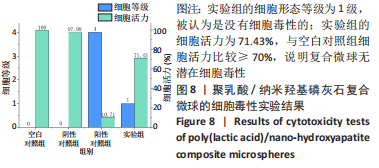
1.4.5 聚乳酸/纳米羟基磷灰石复合微球的细胞毒性 取1 g环氧乙烷灭菌的聚乳酸/纳米羟基磷灰石复合微球样品(或高密度聚乙烯树脂、二乙基二硫代氨基甲酸锌),置入5 mL MEM完全培养基中,于37 ℃培养箱中浸提24 h,制备材料浸提液。
将培养好的小鼠成纤维细胞(BFB细胞库)用0.25%胰蛋白酶溶液消化,制成细胞悬液,调整细胞浓度为105 L-1的细胞悬液,接种于96孔板中,每孔100 μL。置于体积分数5%CO2培养箱中37 ℃培养24 h后,弃去培养液,分4组处理:实验组加入100 μL复合微球浸提液,空白对照组加入100 μL MEM完全培养基,阴性对照组加入100 μL高密度聚乙烯树脂浸提液,阳性对照组加入100 μL二乙基二硫代氨基甲酸锌浸提液,每组设6个平行孔。置于体积分数5%CO2培养箱中37 ℃培养24 h后,弃掉浸提液,每孔中加入50 μL的MTT
(1 mg/mL)溶液,继续培养2 h后,弃掉MTT溶液,每孔中加入100 μL的异丙醇,在摇床中37 ℃、60 r/min振荡培养30 min,在酶标仪以 570 nm 波长测定吸光度值。按照标准《医疗器械生物学评价第5部分:体外细胞毒性试验》中的要求对细胞形态进行观察评级,并评判细胞活力。
细胞存活率(%)=100%×A570e/A570b
式中:A570e为实验组/阴性对照组/阳性对照组吸光度平均值,A570b为空白对照组吸光度平均值
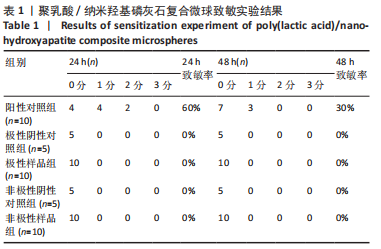
1.4.6 聚乳酸/纳米羟基磷灰石复合微球的致敏反应 取2 g环氧乙烷灭菌的聚乳酸/纳米羟基磷灰石复合微球样品,分别置入10 mL的生理盐水(极性)和棉籽油(非极性)中,于37 ℃培养箱中浸提24 h,分别制备复合微球极性浸提液与非极性浸提液。
按照标准《医疗器械生物学评价第10部分:刺激与皮肤致敏试验》中的方法,选用检疫合格的豚鼠40只,按照体质量大小随机分为对照组和样品组,其中阳性对照组(注射1-氯-2,4-二硝基苯)10只,极性阴性对照组(注射生理盐水)5只,极性样品组(注射复合微球极性浸提液)10只,非极性阴性对照组(注射棉籽油)5只,非极性样品组(注射复合微球非极性浸提液)10只。实验前 24 h,在豚鼠背部肩胛骨附近除毛,除毛面积3 cm×3 cm,在每只动物去毛的肩胛骨内侧部位皮内注射0.1 mL各样品浸提液;皮内诱导阶段未出现刺激反应后,采用面积约8 cm2的敷贴片局部贴敷于每只动物的肩胛骨内侧部位,覆盖诱导注射点;用封闭式包扎带固定敷贴片,并于48 h后除去包扎带和敷贴片;局部诱导阶段后14 d,激发全部实验动物,并于24 h后除去包扎带和敷贴片;除去敷贴片后24,48 h,分别观察各组动物激发部位的皮肤反应,按照标准评分记录结果。将反应记分为1-3分的动物数量除以总动物数量得出致敏率。
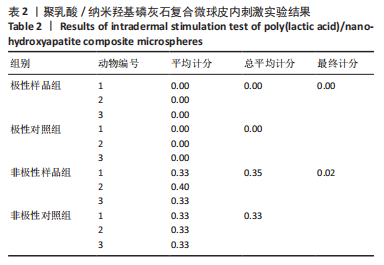
1.4.7 聚乳酸/纳米羟基磷灰石复合微球的皮内反应 取2 g环氧乙烷灭菌的聚乳酸/纳米羟基磷灰石复合微球样品,分别置入10 mL的生理盐水(极性)和棉籽油(非极性)中,于37 ℃培养箱中浸提24 h,分别制备复合微球极性浸提液与非极性浸提液。
在3只新西兰兔脊柱左边上下侧的5个点分别皮内注射0.2 mL的样品极性浸提液(极性样品组)和样品非极性浸提液(非极性样品组),相对应的在右边上下侧则注射0.2 mL的极性溶剂生理盐水(极性对照组)和非极性溶剂棉籽油(非极性对照组)。注射后24,48,72 h,观察各注射部位状况,根据标准《医疗器械生物学评价第10部分:刺激与皮肤致敏试验》中关于皮内刺激实验的记分系统记录实验结果与反应评分。评分方法:将每只动物24,48,72 h后的全部红斑与水肿记分相加,除以15,计算出每只动物记分,并取平均记分;各组平均计分相加得总平均计分。实验样品组总平均记分减去对照组总平均记分可得出样品的最终记分。
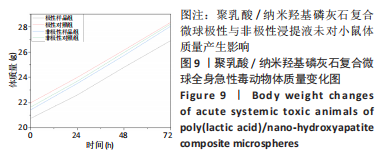
1.4.8 聚乳酸/纳米羟基磷灰石复合微球的全身急性毒反应 取2 g环氧乙烷灭菌的聚乳酸/纳米羟基磷灰石复合微球样品,分别置入10 mL的生理盐水(极性)和棉籽油(非极性)中,于37 ℃培养箱中浸提24 h,分别制备复合微球极性浸提液与非极性浸提液。
给药前,先称量KM小鼠体质量,极性样品组通过尾静脉注射给予复合微球极性浸提液,给药体积均为50 mL/kg,极性对照组尾静脉等量注射生理盐水;非极性样品组通过腹腔注射复合微球非极性浸提液,给药体积均为50 mL/kg,非极性对照组腹腔注射等量棉籽油,每组5只小鼠。注射后,对每只小鼠进行观察,连续观察3 d,每天称体质量1次,观察药物对小鼠体质量的影响。观察后对小鼠进行大体解剖学检查,并记录结果。对死亡和存在大体病理改变的存活动物的器官进行病理组织学检查。
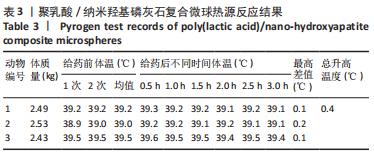
1.4.9 聚乳酸/纳米羟基磷灰石复合微球的热源反应 取15 g紫外灭菌30 min的聚乳酸/纳米羟基磷灰石复合微球样品,加入75 mL生理盐水中,于37 ℃下60 r/min摇床中浸提24 h,制备材料浸提液。
取将新西兰兔3只,在实验前1 h停止给食直到实验完毕,每隔30 min测量体温1次,共测量2次,两次体温差在0.2 ℃内,可用于实验。从新西兰兔耳缘静脉注入样品浸提液,注射剂量为10 mL/kg,每隔0.5 h测量一次体温,共测6次,以测量体温中最高的一次减去正常体温,即为该兔升高温度。当新西兰兔升温为负值时,均以0 ℃计。按照《中国药典》(2020版)1142热源检查法中的要求,3只家兔中,体温升高均低于0.6 ℃,并且3只家兔体温升高总和低于1.3 ℃时,则认为符合热源规定;反之,则需要另取5只兔进行复试,在复试的5只兔中,体温升高0.6 ℃或高于0.6 ℃的家兔不超过1只,并且初试、复试合并8只家兔的体温升高总和为3.5 ℃或低于3.5 ℃,均判定热源检査符合规定,反之则不符合规定。
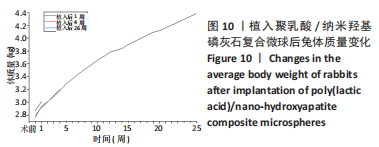
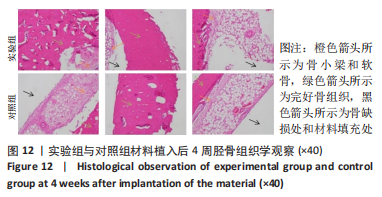
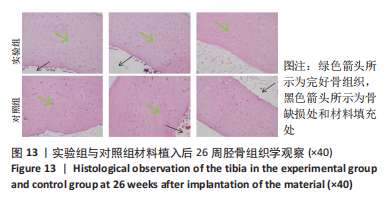
1.4.10 聚乳酸/纳米羟基磷灰石复合微球的骨植入实验 称取0.02 g聚乳酸/纳米羟基磷灰石复合微球倒入直径定制的模具中热压成型,制备出36个直径2 mm、长6 mm大小的圆柱状实验样品植入物(图1);对照品为高密度聚乙烯棒(Hatano Research Instiute,FDSC,H-211)植入物。

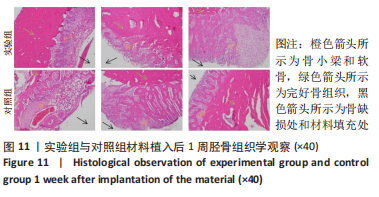
实验24 h前,在12只新西兰兔后肢胫骨部位内侧除去一定面积的被毛,经静脉注射0.08 mL/kg舒泰®50(法国维克有限公司),经肌肉注射0.08 mL/kg速眠新Ⅱ(敦化市圣达动物药品有限公司)进行全身麻醉。将兔后肢部分逐层切开,分离皮肤于皮下组织,划开骨膜,暴露出胫骨,在胫骨上钻孔进行骨制备。钻孔后用植入物填充满,左侧胫骨植入实验样品(实验组),右侧胫骨植入对照样品(对照组),左右各植入3个植入物。在实验期内每天观察实验动物,并记录任何异常发现,包括局部反应及异常行为,每周称体质量一次并记录。植入后1,4,26周,每个时间点以空气栓塞法处死4只兔,解剖后暴露植入部位,检查每一植入部位正常组织结构的改变,切下植入物连带足够的未受影响的骨组织,放入体积分数10%中性缓冲甲醛溶液中固定24 h以上,脱钙后将完整组织包膜与植入物一起包埋,切片,苏木精-伊红染色后进行组织病理学检查。组织学图片,从炎症(多形核白细胞、淋巴细胞、浆细胞、巨噬细胞、巨细胞、坏死)、新血管形成、纤维化、脂肪浸润、创伤性坏死、异物碎片方面进行评分,最后取平均分,将实验组所得评分与对照组所得评分相减,减数值越小刺激越小。
1.5 主要观察指标 聚乳酸/纳米羟基磷灰石复合微球的材料学表征和生物安全性评价。
1.6 统计学分析 所得数据采用SPSS 18.0软件进行统计学分析,数据以x±s表达,组间比较采用t检验,以P < 0.05为差异有显著性意义。该文统计学方法已经武汉理工大学统计学专家审核。