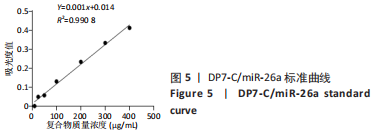
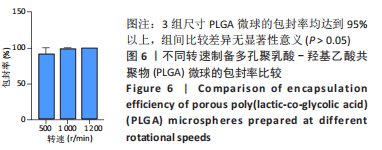
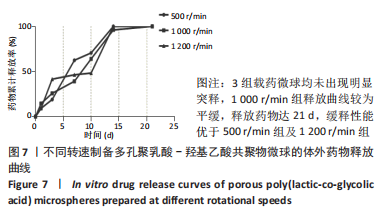
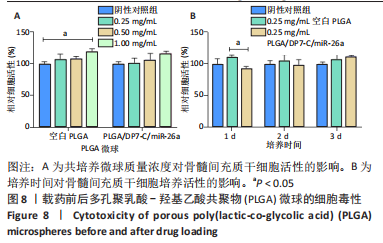
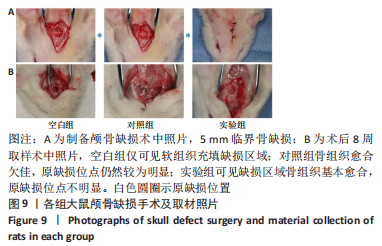
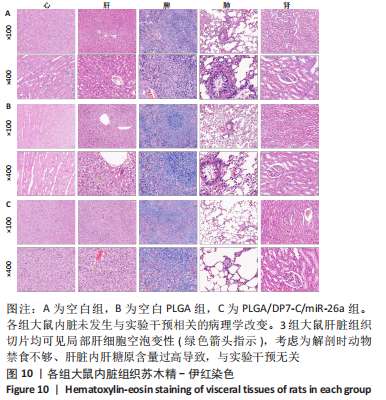
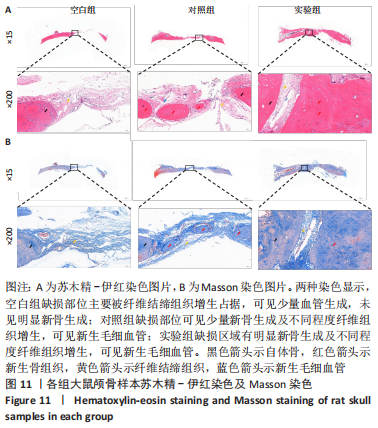
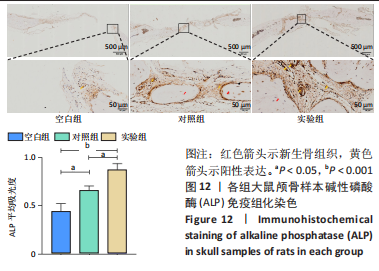
[1] YAN J, LU X, ZHU X, et al. Effects of miR-26a on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by a Mesoporous Silica Nanoparticle - PEI - Peptide System. Int J Nanomedicine. 2020;15:497-511.
[2] WANG Z, ZHANG D, HU Z, et al. MicroRNA-26a-modified adipose-derived stem cells incorporated with a porous hydroxyapatite scaffold improve the repair of bone defects. Mol Med Rep. 2015;12:3345-3350.
[3] PAQUET J, MOYA A, BENSIDHOUM M, et al. Engineered cell-free scaffold with two-stage delivery of miRNA-26a for bone repair. Ann Transl Med. 2016;4(10):3345-3350.
[4] LU J, SONG G, TANG Q, et al. MiR-26a inhibits stem cell-like phenotype and tumor growth of osteosarcoma by targeting Jagged1. Oncogene. 2017;36: 231-241.
[5] CAI Y, YU X, HU S, et al. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinf. 2009;7(4):147-154.
[6] WANG T, XU Z. miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun. 2010;402:186-189.
[7] SU X, LIAO L, SHUAI Y, et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015;6(8):e1851.
[8] ZHANG R, TANG L, TIAN Y, et al. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852.
[9] JIN H, PI J, ZHAO Y, et al. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. 2017;9: 16365-16374.
[10] GARCIA-GARCIA P, REYES R, PEREZ-HERRERO E, et al. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater Sci Eng C Mater Biol Appl. 2020;115:111009.
[11] YAN H, WANG Z, LI L, et al. DOPA-derived electroactive copolymer and IGF-1 immobilized poly(lactic-co-glycolic acid)/hydroxyapatite biodegradable microspheres for synergistic bone repair. Chem Eng J. 2021;416:1385-8947.
[12] LEE JY, KIM SE, YUN YP, et al. Osteogenesis and new bone formation of alendronate-immobilized porous PLGA microspheres in a rat calvarial defect model. J Ind Eng Chem. 2017;52:277-286.
[13] YUAN Y, SHI X, GAN Z, et al. Modification of porous PLGA microspheres by poly-l-lysine for use as tissue engineering scaffolds. Colloids Surf B. 2018; 161:162-168.
[14] NAM MINH-PHUONG T, NHI THAO-NGOC D, NGHI THI-PHUONG N,
et al. Fabrication of injectable bone substitute loading porous simvastatin-loaded poly(lactic-co-glycolic acid) microspheres. Int J Polym Mater Polym Biomater. 2020;69:351-362.
[15] SAH H. Protein instability toward organic solvent/water emulsification: implications for protein microencapsulation into microspheres. PDA J Pharm Sci Technol. 1999;53(1):3-10.
[16] BATTAFARANO G, ROSSI M, DE MARTINO V, et al. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int J Mol Sci. 2021;22:1128.
[17] ZHANG LY, BI Q, ZHAO C, et al. Recent Advances in Biomaterials for the Treatment of Bone Defects. Organogenesis. 2020;16:113-125.
[18] HAMADA T, MATSUBARA H, HIKICHI T, et al. Rat model of an autologous cancellous bone graft. Sci Rep. 2021;11(1):18001.
[19] TOURNIER P, GUICHEUX J, PARÉ A, et al. A partially demineralized allogeneic bone graft: in vitro osteogenic potential and preclinical evaluation in two different intramembranous bone healing models. Sci Rep. 2021;11(1):4907.
[20] BHARADWAZ A, JAYASURIYA AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698.
[21] ZIMMERMANN CE, BORNER BI, HASSE A, et al. Donor site morbidity after microvascular fibula transfer. Clin Oral Investig. 2001;5(4):214-219.
[22] PEREIRA HF, CENGIZ IF, SILVA FS, et al. Scaffolds and coatings for bone regeneration. J Mater Sci Mater Med. 2020;31:27.
[23] SCHMIDT AH. Autologous bone graft: Is it still the gold standard? Injury. 2021; 52 Suppl 2:S18-S22.
[24] MANKIN HJ, HORNICEK FJ, RASKIN KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;(432):210-216.
[25] CHEN CY, CHEN CC, WANG CY, et al. Assessment of the Release of Vascular Endothelial Growth Factor from 3D-Printed Poly-epsilon-Caprolactone/Hydroxyapatite/Calcium Sulfate Scaffold with Enhanced Osteogenic Capacity. Polymers. 2020;12:1455.
[26] REN X, WANG Q, LIU C, et al. Osteogenic ability using porous hydroxyapatite scaffold-based delivery of human placenta-derived mesenchymal stem cells. Exp Ther Med. 2021;22:1091.
[27] LI C, YANG L, REN X, et al. Grooved hydroxyapatite scaffold modulates mitochondria homeostasis and thus promotes osteogenesis in bone mesenchymal stromal cells. Mol Med Rep. 2020;22:2801-2809.
[28] XU T, HE X, CHEN Z, et al. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J Mater Chem B. 2019;7:5648-5660.
[29] LI CL, YANG L, REN XH, et al. Groove structure of porous hydroxyapatite scaffolds (HAS) modulates immune environment via regulating macrophages and subsequently enhances osteogenesis. J Biol Inorg Chem. 2019;24:733-745.
[30] WEI SA, MA JX, XU L, et al. Biodegradable materials for bone defect repair. Mil Med Res. 2020;7:54.
[31] YUAN J, YE Z, ZENG Y, et al. Bifunctional scaffolds for tumor therapy and bone regeneration: Synergistic effect and interplay between therapeutic agents and scaffold materials. Mater Today Bio. 2022;15:100318.
[32] WANG B, FENG C, LIU Y, et al. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: A review. Jpn Dent Sci Rev. 2022;58:233-248.
[33] WAN Z, ZHANG P, LIU Y, et al. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020;101:26-42.
[34] GOMES ME, HOLTORF HL, REIS RL, et al. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:801-809.
[35] CHENG CH, LAI YH, CHEN YW, et al. Immobilization of bone morphogenetic protein-2 to gelatin/avidin-modified hydroxyapatite composite scaffolds for bone regeneration. J Biomater Appl. 2019;33:1147-1156.
[36] CHEN ZY, GAO S, ZHANG YW, et al. Antibacterial biomaterials in bone tissue engineering. J Mater Chem B. 2021;9(11):2594-2612.
[37] SU L, FENG Y, WEI K, et al. Carbohydrate-Based Macromolecular Biomaterials. Chem Rev. 2021;121(18):10950-11029.
[38] BIONDI M, UNGARO F, QUAGLIA F, et al. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229-242.
[39] KANG Y, XU C, MENG LA, et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact Mater. 2022;18:26-41.
[40] JIN S, XIA X, HUANG J, et al. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021;127: 56-79.
[41] BHARADWAZ A, JAYASURIYA AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698.
[42] Maadani AM, Salahinejad E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J Control Release. 2022;351:1-7.
[43] ZHANG BJ, HAN ZW, DUAN K, et al. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J Biomed Mater Res A. 2018;106:95-105.
[44] RAVICHANDRAN R, VENUGOPAL JR, SUNDARRAJAN S, et al. Precipitation of nanohydroxyapatite on PLLA/PBLG/Collagen nanofibrous structures for the differentiation of adipose derived stem cells to osteogenic lineage. Biomaterials. 2012;33:846-855.
|