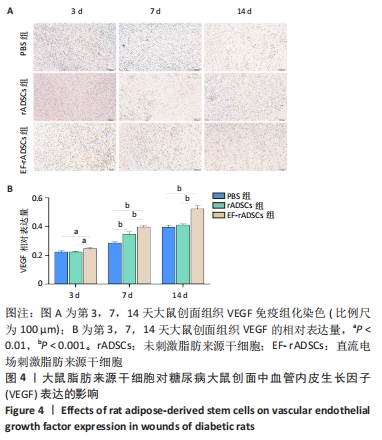
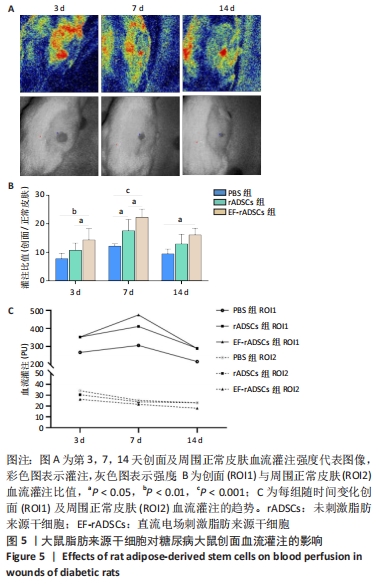
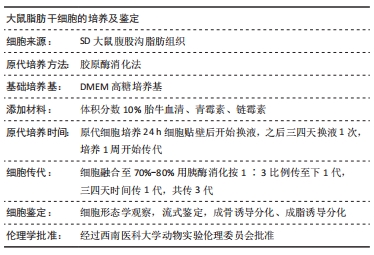
[1] OLSSON M, JÄRBRINK K, DIVAKAR U, et al. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019;27(1):114-125.
[2] SUN X, NI P, WU M, et al. A Clinicoepidemiological Profile of Chronic Wounds in Wound Healing Department in Shanghai. Int J Low Extrem Wounds. 2017;16(1):36-44.
[3] KERN S, EICHLER H, STOEVE J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294-1301.
[4] ENGELA AU, BAAN CC, PEETERS AM, et al. Interaction between adipose tissue-derived mesenchymal stem cells and regulatory T-cells. Cell Transplant. 2013;22(1):41-54.
[5] CAWTHORN WP, SCHELLER EL, MACDOUGALD OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23(6):270-277.
[6] PUISSANT B, BARREAU C, BOURIN P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118-129.
[7] LU C, KOLBENSCHLAG J, NÜSSLER AK, et al. Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life (Basel). 2021;11(11): 1195.
[8] 刘兰,刘星可,刘梦嫦,等.直流电场对人脂肪间充质干细胞增殖和干细胞生物学特性的影响 [J]. 第三军医大学学报,2021,43(11): 1010-1017.
[9] HOLM JS, TOYSERKANI NM, SORENSEN JA. Adipose-derived stem cells for treatment of chronic ulcers: current status. Stem Cell Res Ther. 2018;9(1):142.
[10] SUN Y, SONG L, ZHANG Y, et al. Adipose stem cells from type 2 diabetic mice exhibit therapeutic potential in wound healing. Stem Cell Res Ther. 2020;11(1):298.
[11] STOJANOVIĆ S, NAJMAN S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int J Mol Sci. 2019;20(7):1671.
[12] KLAR AS, MICHALAK-MIĆKA K, BIEDERMANN T, et al. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int. 2018;34(2): 129-135.
[13] ZOMER HD, JEREMIAS TDS, RATNER B, et al. Mesenchymal stromal cells from dermal and adipose tissues induce macrophage polarization to a pro-repair phenotype and improve skin wound healing. Cytotherapy. 2020;22(5):247-260.
[14] MAZINI L, ROCHETTE L, ADMOU B, et al. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21(4):1306.
[15] ZHOU X, NING K, LING B, et al. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019;28(21):1463-1472.
[16] ALEXANDRUSHKINA N, NIMIRITSKY P, EREMICHEV R, et al. Cell Sheets from Adipose Tissue MSC Induce Healing of Pressure Ulcer and Prevent Fibrosis via Trigger Effects on Granulation Tissue Growth and Vascularization. Int J Mol Sci. 2020;21(15):5567.
[17] HUANG SP, HUANG CH, SHYU JF, et al. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci. 2013;20(1):51.
[18] GENG Y, YANG J, LI S, et al. Chyloid Fat Carried Adipose-Derived Mesenchymal Stem Cells Accelerate Wound Healing Via Promoting Angiogenesis. Ann Plast Surg. 2021;87(4):472-477.
[19] NAMBU M, KISHIMOTO S, NAKAMURA S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62(3):317-321.
[20] AJIT A, SANTHOSH KUMAR TR, KRISHNAN LK. Engineered Human Adipose-Derived Stem Cells Inducing Endothelial Lineage and Angiogenic Response. Tissue Eng Part C Methods. 2019;25(3):148-159.
[21] QIAN L, PI L, FANG BR, et al. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101(9):1254-1266.
[22] RODRIGUEZ J, BOUCHER F, LEQUEUX C, et al. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Res Ther. 2015;6:241.
[23] LOVE MR, PALEE S, CHATTIPAKORN SC, et al. Effects of electrical stimulation on cell proliferation and apoptosis. J Cell Physiol. 2018; 233(3):1860-1876.
[24] BROUGHTON G 2ND, JANIS JE, ATTINGER CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
[25] 颜洪.着眼瘢痕防治,促进创面愈合 [J].第三军医大学学报,2021, 43(11):997-1002.
[26] WILKINSON HN, HARDMAN MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223.
[27] WU Y, CHEN L, SCOTT PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648-2659.
[28] NIE C, YANG D, MORRIS SF. Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses. 2009;72(6):679-682.
[29] HUANG S, HU Z, WANG P, et al. Rat epidermal stem cells promote the angiogenesis of full-thickness wounds. Stem Cell Res Ther. 2020; 11(1):344.
[30] DOMINICI M, LE BLANC K, MUELLER I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8(4):315-317.
[31] BAO P, KODRA A, TOMIC-CANIC M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347-358.
[32] BATES DO, JONES RO. The role of vascular endothelial growth factor in wound healing. Int J Low Extrem Wounds. 2003;2(2):107-120.
[33] JOHNSON KE, WILGUS TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle). 2014;3(10):647-661.
[34] CHUNG AS, FERRARA N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563-584.
[35] ONG HT, DILLEY RJ. Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev. 2018;44:69-79.
[36] ZHANG P, LU J, JING Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017; 49(2):106-116.
[37] YANG M, SHENG L, ZHANG TR, et al. Stem cell therapy for lower extremity diabetic ulcers: where do we stand? Biomed Res Int. 2013; 2013:462179.
[38] SORG H, TILKORN DJ, MIRASTSCHIJSKI U, et al. Panta Rhei: Neovascularization, Angiogenesis and Nutritive Perfusion in Wound Healing. Eur Surg Res. 2018;59(3-4):232-241.
[39] REGE A, THAKOR NV, RHIE K, et al. In vivo laser speckle imaging reveals microvascular remodeling and hemodynamic changes during wound healing angiogenesis. Angiogenesis. 2012;15(1):87-98.
|