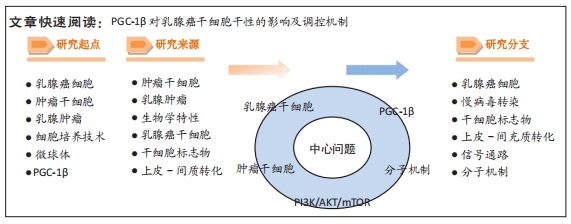
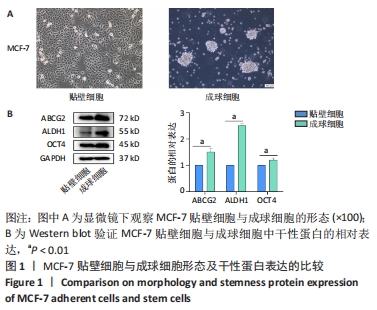
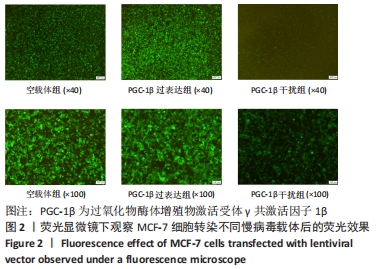
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
[2] 国家肿瘤质控中心乳腺癌专家委员会, 中国抗癌协会乳腺癌专业委员会, 中国抗癌协会肿瘤药物临床研究专业委员会. 中国晚期乳腺癌规范诊疗指南(2020版)[J].中华肿瘤杂志,2020,42(10):781-797.
[3] HE L, WICK N, GERMANS SK, et al. The Role of Breast Cancer Stem Cells in Chemoresistance and Metastasis in Triple-Negative Breast Cancer. Cancers (Basel). 2021;13(24):6209.
[4] WU HJ, CHU PY. Epigenetic Regulation of Breast Cancer Stem Cells Contributing to Carcinogenesis and Therapeutic Implications. Int J Mol Sci. 2021;22(15):8113.
[5] MARIMA R, FRANCIES FZ, HULL R, et al. MicroRNA and Alternative mRNA Splicing Events in Cancer Drug Response/Resistance: Potent Therapeutic Targets. Biomedicines. 2021;9(12):1818.
[6] ZENG X, LIU C, YAO J, et al. Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol Res. 2021; 163:105320.
[7] PICCININ E, PERES C, BELLAFANTE E, et al. Hepatic peroxisome proliferator-activated receptor γ coactivator 1β drives mitochondrial and anabolic signatures that contribute to hepatocellular carcinoma progression in mice. Hepatology. 2018;67(3):884-898.
[8] KELLY DP, SCARPULLA RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357-368.
[9] DUCHEIX S, VEGLIANTE MC, VILLANI G, et al. Is hepatic lipogenesis fundamental for NAFLD/NASH? A focus on the nuclear receptor coactivator PGC-1β. Cell Mol Life Sci. 2016;73(20):3809-3822.
[10] BELLAFANTE E, MURZILLI S, SALVATORE L, et al. Hepatic-specific activation of peroxisome proliferator-activated receptor γ coactivator-1β protects against steatohepatitis. Hepatology. 2013;57(4):1343-1356.
[11] LI G, TAN X, ZHANG B, et al. Hengshun Aromatic Vinegar Improves Glycolipid Metabolism in Type 2 Diabetes Mellitus via Regulating PGC-1α/PGC-1β Pathway. Front Pharmacol. 2021;12:641829.
[12] EDLING CE, FAZMIN IT, CHADDA KR, et al. Ageing in Pgc-1β-/- mice modelling mitochondrial dysfunction induces differential expression of a range of genes regulating ventricular electrophysiology. Biosci Rep. 2019;39(4):BSR20190127.
[13] ZHAI M, LIU Z, ZHANG B, et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1β: In vivo and in vitro studies. J Pineal Res. 2017;63(3). doi: 10.1111/jpi.12433.
[14] WANG L, LIU Q, LI F, et al. Apoptosis induced by PGC-1β in breast cancer cells is mediated by the mTOR pathway. Oncol Rep. 2013;30(4): 1631-1638.
[15] CAO J, WANG X, WANG D, et al. PGC-1β cooperating with FOXA2 inhibits proliferation and migration of breast cancer cells. Cancer Cell Int. 2019;19:93.
[16] RUHEN O, QU X, JAMALUDDIN MFB, et al. Dynamic Landscape of Extracellular Vesicle-Associated Proteins Is Related to Treatment Response of Patients with Metastatic Breast Cancer. Membranes (Basel). 2021;11(11):880.
[17] MALEBARI AM, WANG S, GREENE TF, et al. Synthesis and Antiproliferative Evaluation of 3-Chloroazetidin-2-ones with Antimitotic Activity: Heterocyclic Bridged Analogues of Combretastatin A-4. Pharmaceuticals (Basel). 2021;14(11):1119.
[18] NAZ F, SHI M, SAJID S, et al. Cancer stem cells: a major culprit of intra-tumor heterogeneity. Am J Cancer Res. 2021;11(12):5782-5811.
[19] ZHANG T, ZHOU H, WANG K, et al. Role, molecular mechanism and the potential target of breast cancer stem cells in breast cancer development. Biomed Pharmacother. 2022;147:112616.
[20] DENG J, BAI X, FENG X, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19(1):618.
[21] BAHENA-OCAMPO I, ESPINOSA M, CEBALLOS-CANCINO G, et al. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016;17(5):648-658.
[22] VANDER HEIDEN MG, CANTLEY LC, THOMPSON CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033.
[23] ANDRZEJEWSKI S, KLIMCAKOVA E, JOHNSON RM, et al. PGC-1α Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017;26(5):778-787.e5.
[24] ANDERSON AS, ROBERTS PC, FRISARD MI, et al. Ovarian tumor-initiating cells display a flexible metabolism. Exp Cell Res. 2014;328(1):44-57.
[25] GLEYZER N, SCARPULLA RC. Activation of a PGC-1-related coactivator (PRC)-dependent inflammatory stress program linked to apoptosis and premature senescence. J Biol Chem. 2013;288(12):8004-8015.
[26] PUIGSERVER P, WU Z, PARK CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6): 829-839.
[27] SINGH F, ZOLL J, DUTHALER U, et al. PGC-1β modulates statin-associated myotoxicity in mice. Arch Toxicol. 2019;93(2):487-504.
[28] CHAMBERS JM, WINGERT RA. PGC-1α in Disease: Recent Renal Insights into a Versatile Metabolic Regulator. Cells. 2020;9(10):2234.
[29] VICTORINO VJ, BARROSO WA, ASSUNÇÃO AK, et al. PGC-1β regulates HER2-overexpressing breast cancer cells proliferation by metabolic and redox pathways. Tumour Biol. 2016;37(5):6035-6044.
[30] LI Y, KASIM V, YAN X, et al. Yin Yang 1 facilitates hepatocellular carcinoma cell lipid metabolism and tumor progression by inhibiting PGC-1β-induced fatty acid oxidation. Theranostics. 2019;9(25):7599-7615.
[31] WANG H, YAN X, JI LY, et al. miR-139 Functions as An Antioncomir to Repress Glioma Progression Through Targeting IGF-1 R, AMY-1, and PGC-1β. Technol Cancer Res Treat. 2017;16(4):497-511.
[32] LAMBERT AW, WEINBERG RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21(5):325-338.
[33] DASGUPTA A, SAWANT MA, KAVISHWAR G, et al. AECHL-1 targets breast cancer progression via inhibition of metastasis, prevention of EMT and suppression of Cancer Stem Cell characteristics. Sci Rep. 2016;6:38045.
[34] GONG C, ZOU J, ZHANG M, et al. Upregulation of MGP by HOXC8 promotes the proliferation, migration, and EMT processes of triple-negative breast cancer. Mol Carcinog. 2019;58(10):1863-1875.
[35] ACUÑA RA, VARAS-GODOY M, HERRERA-SEPULVEDA D, et al. Connexin46 Expression Enhances Cancer Stem Cell and Epithelial-to-Mesenchymal Transition Characteristics of Human Breast Cancer MCF-7 Cells. Int J Mol Sci. 2021;22(22):12604.
[36] LI H, PREVER L, HIRSCH E, et al. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). 2021;13(14):3517.
[37] WANG L, YANG M, JIN H. PI3K/AKT phosphorylation activates ERRα by upregulating PGC‑1α and PGC‑1β in gallbladder cancer. Mol Med Rep. 2021;24(2):613.
[38] MCKENNA M, MCGARRIGLE S, PIDGEON GP. The next generation of PI3K-Akt-mTOR pathway inhibitors in breast cancer cohorts. Biochim Biophys Acta Rev Cancer. 2018;1870(2):185-197.
[39] LEE YR, CHEN M, PANDOLFI PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19(9):547-562.
[40] JUNG MJ, RHO JK, KIM YM, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209-221.
[41] LUONGO F, COLONNA F, CALAPÀ F, et al. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers (Basel). 2019;11(8):1076.
|