| [1]刘兴漠,项禹诚,潘滔,等.胶原/羟基磷灰石一体化支架修复关节软骨缺损的机制研究[J].中国康复理论与实践,2012,18(1): 37-40.[2]刘兴漠,项禹诚,孙青,等.周期性张应力对骨性关节炎软骨细胞p38MAPK表达及其磷酸化的影响[J].中国病理生理杂志,2012, 28(2):362-365,370.[3]Beris AE, Lykissas MG, Papageorgiou CD,et al. Advances in articular cartilage repair.Injury. 2005;36 Suppl 4:S14-23.[4]晏丹,周广东,曹谊林.关节软骨生化结构及其与力学性能关系研究进展[J].上海交通大学学报:医学版,2009,29 (3):341-345.[5]彭雪林,张利,李玉宝,等.关节软骨缺损修复的研究与进展[J].中国组织工程研究与临床康复,2007,11 (2):336-339.[6]McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology.Sports Med Arthrosc. 2008; 16(4): 196-201.[7]Martinek V, Ueblacker P, Imhoff AB.Current concepts of gene therapy and cartilage repair.J Bone Joint Surg Br. 2003;85(6): 782-788.[8]马树强,姬海鹏,王坤正,等.骨-骨膜复合组织与软骨下钻孔治疗关节软骨缺损的实验研究[J].陕西医学杂志,2004,33(5) : 390-392.[9]刘尚礼,李卫平,宋卫东.软骨细胞移植治疗关节软骨缺损[J].广州医药,2000,31(2): 1-4.[10]Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337-4351.[11]刘兴漠,项禹诚,麦海民,等.关节软骨-骨一体化修复体修复全层关节软骨缺损的实验研究[J].中华骨科杂志,2011,31(4): 365-371.[12]Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358-1375.[13]Han J, Lee JD, Bibbs L,et al. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells.Science. 1994;265(5173):808-811.[14]Lee JC, Laydon JT, McDonnell PC,et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739-746.[15]Rouse J, Cohen P, Trigon S,et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins.Cell. 1994;78(6):1027-1037.[16]Freshney NW, Rawlinson L, Guesdon F,et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27.Cell. 1994;78(6):1039-1049.[17]Wada Y, Shimada K, Sugimoto K,et al. Novel p38 mitogen-activated protein kinase inhibitor R-130823 protects cartilage by down-regulating matrix metalloproteinase-1,-13 and prostaglandin E2 production in human chondrocytes.Int Immunopharmacol. 2006;6(2):144-155.[18]Link DP, Strandberg JD, Virmani R,et al. Histopathologic appearance of arterial occlusions with hydrogel and polyvinyl alcohol embolic material in domestic swine. J Vasc Interv Radiol. 1996;7(6):897-905.[19]Erskine RM, Jones DA, Williams AG,et al. Resistance training increases in vivo quadriceps femoris muscle specific tension in young men.Acta Physiol (Oxf). 2010;199(1):83-89.[20]D'Antona G, Lanfranconi F, Pellegrino MA,et al. Skeletal muscle hypertrophy and structure and function of skeletal muscle fibres in male body builders. J Physiol. 2006;570(Pt 3):611-627.[21]Hinz B, Brune K. Pain and osteoarthritis: new drugs and mechanisms. Curr Opin Rheumatol. 2004;16(5): 628-633.[22]Poole CA.Articular cartilage chondrons: form, function and failure. J Anat. 1997;191 ( Pt 1):1-13.[23]Thiede RM, Lu Y, Markel MD. A review of the treatment methods for cartilage defects.Vet Comp Orthop Traumatol. 2012;25(4):263-272.[24]Hurtig M, Pearce S, Warren S,et al. Arthroscopic mosaic arthroplasty in the equine third carpal bone.Vet Surg. 2001; 30(3):228-239.[25]Solheim E, Hegna J, Oyen J,et al. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17(1):84-87.[26]Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191-195.[27]Stevenson S, Dannucci GA, Sharkey NA,et al. The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1989;71(9):1297-1307.[28]Danisovic L, Varga I, Zamborsky R,et al.The tissue engineering of articular cartilage: cells, scaffolds and stimulating factors.Exp Biol Med (Maywood). 2012;237 (1):10-17. [29]Nah SS, Choi IY, Yoo B,et al. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581(9):1928-1932.[30]Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12(6):485-496.[31]Wada Y, Shimada K, Sugimoto K,et al. Novel p38 mitogen-activated protein kinase inhibitor R-130823 protects cartilage by down-regulating matrix metalloproteinase-1,-13 and prostaglandin E2 production in human chondrocytes.Int Immunopharmacol. 2006;6(2):144-155.[32]Holm L, van Hall G, Rose AJ,et al. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(2):E257-269.[33]Mackey AL, Donnelly AE, et al. Turpeenniemi-Hujanen T,Skeletal muscle collagen content in humans after high-force eccentric contractions. J Appl Physiol. 2004;97(1):197-203.[34]Moore DR, Phillips SM, Babraj JA,et al. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions.Am J Physiol Endocrinol Metab. 2005;288(6): E1153-1159.[35]Hollander MS, Baker BA, Ensey J,et al. Effects of age and glutathione levels on oxidative stress in rats after chronic exposure to stretch-shortening contractions. Eur J Appl Physiol. 2010;108(3):589-597.[36]Baker BA, Hollander MS, Kashon ML,et al. Effects of glutathione depletion and age on skeletal muscle performance and morphology following chronic stretch-shortening contraction exposure. Eur J Appl Physiol. 2010;108(3):619-630.[37]Warner SE, Sanford DA, Becker BA,et al. Botox induced muscle paralysis rapidly degrades bone. Bone. 2006;38(2): 257-264. [38]van Osch GJ, van der Kraan PM, van Valburg AA,et al.The relation between cartilage damage and osteophyte size in a murine model for osteoarthritis in the knee. Rheumatol Int. 1996;16(3):115-119.[39]Liacini A, Sylvester J, Li WQ,et al. Mithramycin downregulates proinflammatory cytokine-induced matrix metalloproteinase gene expression in articular chondrocytes. Arthritis Res Ther. 2005;7(4):R777-783. [40]Scanzello CR, Umoh E, Pessler F,et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease.Osteoarthritis Cartilage. 2009;17(8):1040-1048.[41]Murphy G, Atkinson S, Ward R,et al.The role of plasminogen activators in the regulation of connective tissue metalloproteinases.Ann N Y Acad Sci. 1992;667:1-12. [42]吴宏斌,杜靖远,胡勇.兔前交叉韧带切断骨关节炎模型中MMP-1 MMP-13及TIMP-1的mRNA表达研究[J].中华风湿病学杂志, 2002,6(3):169-173. [43]李强,唐际存,王锐英,等.创伤性关节炎软骨中基质金属蛋白酶13及组织特异性抑制剂1的表达[J].中国组织工程研究,2012,16(4): 695-698.[44]Flannery CR, Little CB, Caterson B,et al. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999;18(3): 225-237.[45]Lee JH, Fitzgerald JB, Dimicco MA,et al. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8): 2386-2395. [46]Clegg DO, Reda DJ, Harris CL,et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808.[47]吴并生,薛华新,刘晋,等.超短波对家兔膝关节骨关节炎形成过程的影响[J].中华物理医学与康复杂志,2003,25(1):7-10.[48]俞晓杰,吴毅.运动疗法在膝关节骨关节中的应用[J].中华物理医学与康复杂志,2005,27(9):559-561.[49]Gill TJ.The treatment of articular cartilage defects using microfracture and debridement. Am J Knee Surg. 2000; 13(1):33-40.[50]Malemud CJ, Islam N, Haqqi TM. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies.Cells Tissues Organs. 2003;174(1-2):34-48. |
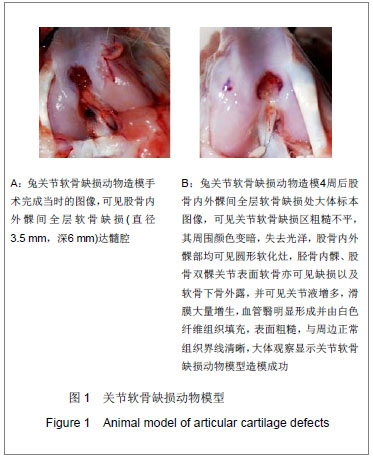
.jpg)
.jpg)
.jpg) 随着社会的发展,各种原因导致的软骨缺损的发病率逐年上升,关节软骨因缺乏直接的血液供应、淋巴循环和神经支配,当外伤或疾病而发生缺损时通常自身修复能力有限或不能自行修复[6-7]。关节创伤和关节疾病等均可导致关节软骨的缺损,临床上较为常见[8],在创伤、炎症等病因影响下,关节损伤后常常会引起关节功能障碍,导致骨关节炎的发生[9]。软骨缺损病变过程的主要病理特征是软骨细胞凋亡及基质的破坏[10]。有研究证实异常的应力刺激下,软骨细胞出现功能失调及下降,基质合成减少,引发关节退变[11]。有研究证实软骨细胞和基质的结构和功能受温度、化学、pH值、生物、应力等多种因素影响[12]。应力是软骨细胞所处的重要微环境之一,应力主要通过软骨细胞和基质内的力敏感细胞起作用[13]。现临床上人们已将应力用于治疗骨折、关节软骨缺损、骨不连等,并取得一定疗效,说明应力可促进骨及软骨的再生[14-15]。机械载荷对软骨结构、质量起重要调节作用,它能引起与骨生成有关的成骨、软骨以及骨细胞的反应[16-17],即应力可以促进骨生成相关细胞的增殖、增加生长因子的分泌以及促进细胞外基质的合成等。近年研究证明,细胞表面离子通道在细胞膜受到力学作用数秒或数分钟后可引起G蛋白活化、第二信使释放、蛋白质磷酸化、基因表达改变、细胞骨架变化、生长因子分泌、细胞和细胞外基质黏附重建等[18-20]。在关节软骨缺损的发生和发展过程中,力学因素的作用不容忽视。有研究发现软骨胶原网中,基质分子遵循利于力学功能的原则在胶原网结构中沉积[21],这说明当力学信号的力学效应通过软骨细胞的某种途径进入细胞核中,再进一步调控各种相关基因表达,从而产生生物学效应,即力学信号-生物学信号-相关基因表达-生物学效应,见图3。
随着社会的发展,各种原因导致的软骨缺损的发病率逐年上升,关节软骨因缺乏直接的血液供应、淋巴循环和神经支配,当外伤或疾病而发生缺损时通常自身修复能力有限或不能自行修复[6-7]。关节创伤和关节疾病等均可导致关节软骨的缺损,临床上较为常见[8],在创伤、炎症等病因影响下,关节损伤后常常会引起关节功能障碍,导致骨关节炎的发生[9]。软骨缺损病变过程的主要病理特征是软骨细胞凋亡及基质的破坏[10]。有研究证实异常的应力刺激下,软骨细胞出现功能失调及下降,基质合成减少,引发关节退变[11]。有研究证实软骨细胞和基质的结构和功能受温度、化学、pH值、生物、应力等多种因素影响[12]。应力是软骨细胞所处的重要微环境之一,应力主要通过软骨细胞和基质内的力敏感细胞起作用[13]。现临床上人们已将应力用于治疗骨折、关节软骨缺损、骨不连等,并取得一定疗效,说明应力可促进骨及软骨的再生[14-15]。机械载荷对软骨结构、质量起重要调节作用,它能引起与骨生成有关的成骨、软骨以及骨细胞的反应[16-17],即应力可以促进骨生成相关细胞的增殖、增加生长因子的分泌以及促进细胞外基质的合成等。近年研究证明,细胞表面离子通道在细胞膜受到力学作用数秒或数分钟后可引起G蛋白活化、第二信使释放、蛋白质磷酸化、基因表达改变、细胞骨架变化、生长因子分泌、细胞和细胞外基质黏附重建等[18-20]。在关节软骨缺损的发生和发展过程中,力学因素的作用不容忽视。有研究发现软骨胶原网中,基质分子遵循利于力学功能的原则在胶原网结构中沉积[21],这说明当力学信号的力学效应通过软骨细胞的某种途径进入细胞核中,再进一步调控各种相关基因表达,从而产生生物学效应,即力学信号-生物学信号-相关基因表达-生物学效应,见图3。 .jpg) 关节软骨缺损修复治疗是临床的重大难题之一,随着科学技术的发展,关于关节软骨缺损的修复取得了一定的进展,但尚无最佳治疗方法。目前临床上主要治疗方法包括软骨下骨钻孔、微骨折术、软骨镶嵌成形术、同种异体关节软骨移植等,上述方法均有其局限
关节软骨缺损修复治疗是临床的重大难题之一,随着科学技术的发展,关于关节软骨缺损的修复取得了一定的进展,但尚无最佳治疗方法。目前临床上主要治疗方法包括软骨下骨钻孔、微骨折术、软骨镶嵌成形术、同种异体关节软骨移植等,上述方法均有其局限