中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (13): 2105-2113.doi: 10.12307/2024.108
• 干细胞综述 stem cell review • 上一篇 下一篇
外泌体及预处理方式对牙髓再生的作用
杨润泽1,2,王 玮2,陈 三1,周学东3,吴家媛1
- 1遵义医科大学口腔医学院,遵义医科大学附属口腔医院,贵州省遵义市 563000;2军事口腔医学国家重点实验室,国家口腔疾病临床医学研究中心,陕西省口腔疾病临床医学研究中心,空军军医大学第三附属医院牙体牙髓病科,陕西省西安市 710032;3四川大学华西口腔医院,四川省成都市 610041
-
收稿日期:2023-01-29接受日期:2023-03-02出版日期:2024-05-08发布日期:2023-08-29 -
通讯作者:吴家媛,女,博士,教授,硕士生导师,遵义医科大学附属口腔医院,贵州省遵义市 563000 周学东,教授,博士,四川大学华西口腔医院,四川省成都市 610041 -
作者简介:杨润泽,男,1992年生,四川省成都市人,汉族,遵义医科大学在读硕士,主要从事口腔微生态与口腔感染性疾病的防治研究。 -
基金资助:陕西省重点研发计划项目(2021KW-61),项目负责人:王玮;贵州省科技计划项目(ZK[2022]-638),项目负责人:吴家媛
Effect of exosomes and the preconditioning method on pulp regeneration
Yang Runze1, 2, Wang Wei2, Chen San1, Zhou Xuedong3, Wu Jiayuan1
- 1Hospital/School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; 2State Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases & Shaanxi Key Laboratory of Stomatology, Department of Operative Dentistry and Endodontics of Third Affiliated Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China; 3West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
-
Received:2023-01-29Accepted:2023-03-02Online:2024-05-08Published:2023-08-29 -
Contact:Wu Jiayuan, MD, Professor, Master’s supervisor, Hospital/School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China Zhou Xuedong, MD, Professor, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China -
About author:Yang Runze, Master candidate, Hospital/School of Stomatology, Zunyi Medical University, Zunyi 563000, Guizhou Province, China; State Key Laboratory of Military Stomatology & National Clinical Research Center for Oral Diseases & Shaanxi Key Laboratory of Stomatology, Department of Operative Dentistry and Endodontics of Third Affiliated Hospital of Air Force Medical University, Xi’an 710032, Shaanxi Province, China -
Supported by:Key Research and Development Project of Shaanxi Province, No, 2021KW-61 (to WW); Science and Technology Planning Project of Guizhou Province, No. ZK[2022]-638 (to WJY)
摘要:
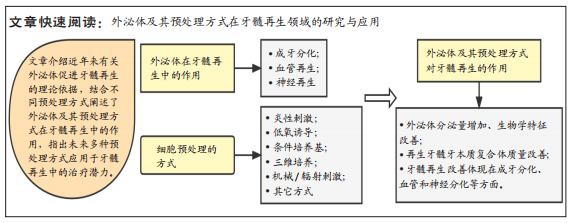
文题释义:
经细胞预处理分泌的外泌体:细胞的培养环境高度影响着其分泌的外泌体生物学特性,通过改变细胞的培养环境以达到改善外泌体的功能特性的目的,从而优化外泌体在组织再生等领域中的治疗效果与应用。牙髓再生:通过调控干细胞的增殖、迁移和分化促进牙髓血管和神经的生成以及牙髓和牙本质样组织的形成,最终达到重建受损牙髓组织的结构和功能特性的目的。
背景:现有研究已经证实外泌体可有效促进牙髓再生,而经预处理来源的外泌体其生物学功能和特性会发生显著改变,对细胞的增殖、迁移和成牙分化产生不同的影响。
目的:探讨外泌体及其预处理方式在牙髓再生领域的应用现状,归纳和总结影响外泌体发挥作用的预处理方式,并阐述外泌体及其预处理方式对牙髓再生的作用。方法:检索万方、中国知网、PubMed和Web of Science数据库中2006-2022年发表的相关文献,以“外泌体,牙髓再生,预处理方式”等为中文检索词,以“Exosomes,Pulp regeneration,Preconditioning method ”等为英文检索词进行检索,共纳入78篇文献进行综述分析。
结果与结论:①外泌体具有良好的生物相容性、低免疫原性和无细胞毒性等优势,可以通过促进干细胞成牙、成神经和成血管化进而诱导牙髓组织的新生。②经预处理衍生的外泌体可以增强对组织的修复和再生能力,并对再生牙髓的质量有显著影响。③目前应用在牙髓再生领域中的预处理方式包括炎症刺激、低氧诱导、条件培养基和三维培养,其分泌的外泌体均能有效改善再生牙髓的质量,但是不同的预处理方式对牙髓再生的具体效果和机制在未来尚需探索。
https://orcid.org/0000-0003-0362-5957(杨润泽);https://orcid.org/0000-0001-5300-1267(吴家媛);https://orcid.org/0000-0002-9319-7302(周学东)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号:
引用本文
杨润泽, 王 玮, 陈 三, 周学东, 吴家媛. 外泌体及预处理方式对牙髓再生的作用[J]. 中国组织工程研究, 2024, 28(13): 2105-2113.
Yang Runze, Wang Wei, Chen San, Zhou Xuedong, Wu Jiayuan. Effect of exosomes and the preconditioning method on pulp regeneration[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2105-2113.
2.1.1 外泌体的特点 外泌体是由细胞所分泌的一种具有生物活性的微小囊泡,直径为50-150 nm,具有化学性质稳定、组织选择性高、能高效修复受损组织、易于保存、方便运输等特点[18-19]。外泌体由母细胞释放后,通过靶向配体/受体相互识别、内吞到受体细胞或与受体细胞质膜融合等方式,进入受体靶细胞,并将其有效信号分子释放,从而触发了相应的生物学效应[20]。外泌体携带有脂质、蛋白质、DNA、mRNAs、miRNA、siRNA和lncRNA等信号分子,其本身脂质双分子膜的结构可以使其携带的生物信息不被降解,且可以通过血脑屏障等生物膜从而发挥重要作用[5,7,21-22]。研究发现不同细胞来源或同一细胞不同状态下分泌的外泌体功能特性有所不同,且它们所携带的生物学信息与其所处的微环境紧密相关[23]。因此,基于以上特性,外泌体在组织修复再生、药物递送载体和疾病治疗等方面受到了广大研究者的青睐。
2.1.2 外泌体对牙髓再生的作用 近年来,在牙髓再生领域中,外泌体已经被证实可以有效地促进牙髓牙本质复合体的再生,但不同细胞来源的外泌体其生物学特性以及功能不同。研究表明,间充质细胞来源的外泌体诱导表皮细胞产生了基底膜的复合物、釉原蛋白和成釉蛋白,而表皮来源的外泌体促进了间充质细胞的成牙分化和矿化[24]。牙龈组织来源的外泌体促进施万细胞的增殖和迁移,调控相关基因的表达并促进了神经的再生[25]。牙齿组织来源的外泌体在牙髓发生发展的过程中发挥着重要作用。牙髓细胞来源的外泌体能促进牙髓干细胞的成牙分化,当其与牙髓干细胞复合后植入裸鼠皮下,在牙齿切片上观察到了牙髓样组织的再生[4]。因此将不同细胞来源的外泌体用于牙髓再生领域,实现牙髓血管、神经以及牙髓牙本质再生是一项很有前景的研究。
牙髓干细胞分离并提取于恒牙牙髓组织,其具有良好的增殖能力和定向分化能力,是目前应用于牙髓再生领域中的主要种子细胞。有学者研究发现,牙髓干细胞将其衍生的外泌体内吞后检测到其牙本质磷蛋白、牙本质基质蛋白1、碱性磷酸酶及RUNT相关转录因子2(Runt-related transcript factor,RUNX2)等成牙分化相关因子表达上调,并发现牙髓干细胞的成牙分化中转化生长因子β1/Smad信号通路被激活[26]。HUANG等[4]研究再次证实牙髓干细胞来源外泌体上调了牙髓干细胞的牙本质涎磷蛋白、碱性磷酸酶及RUNX2等成牙分化相关因子的表达,并在裸鼠皮下观察到牙髓样组织的形成,且通过抑制P38丝裂原活化蛋白激酶信号通路,促进了血管生成。上述研究证实牙髓干细胞分泌的外泌体能够被细胞内吞后促进相关分化等作用且在此过程中通过不同的信号通路促进成牙分化和血管的生成,相关因子也进行表达,进而诱导牙髓组织的再生。
牙髓具有丰富的血管组织,充足的血供是牙髓发挥功能作用的前提基础。血管的生成包含多种生长因子参与,如成纤维细胞生长因子、血管内皮生长因子、表皮生长因子等,其中血管内皮生长因子与血管生成相关性最高,其可以促进血管内皮细胞的增殖和有丝分裂从而促进血管和侧支循环形成。XIAN等[27]研究表明,人类牙髓细胞来源的外泌体可上调血管内皮生长因子等多种促血管生成因子表达,促进人脐静脉内皮细胞增殖和分化。根尖牙乳头干细胞是从未成熟的根尖中分离并提取的,其成牙功能较牙髓干细胞优异。ZHUANG等[28]将根尖牙乳头干细胞来源的外泌体作用于骨髓间充质干细胞,发现牙本质涎磷蛋白表达上调以及矿化结节形成显著增加,将两者复合物移植到裸鼠皮下,最终观察到牙髓样组织生成,且管壁新形成的牙本质沉积在原有牙本质层上,证明根尖牙乳头干细胞来源外泌体在成牙分化方面具有较好的表现。LIN等[14]检测到在根尖牙乳头干细胞将其衍生的外泌体内吞后,促进了血管生成,表明根尖牙乳头干细胞来源外泌体不仅改善了特异性牙本质的生成且能够促进血管的形成,推测其机制可能通过miR-126表达上调且通过激活ERK信号通路实现。脱落乳牙牙髓干细胞也被称为年轻的牙髓干细胞,其拥有更强的增殖和多向分化潜能,在成神经成血管方面较牙髓干细胞突出。一项研究显示,脱落乳牙牙髓干细胞聚合体衍生的外泌体促进了血管的新生[29],其机制被发现是外泌体分泌的miR-26a通转化生长因子β/Smad2/3信号通路促进了该过程,在体内研究结果中观察到牙髓样组织的生成。以往相关体内研究也进一步观察到在新生主血管周围存在大量毛细血管和粗壮的铰链状神经组织[30-31]。
上述细胞来源的外泌体被大量应用在牙髓再生中,另有研究发现其他细胞来源外泌体也具有相似促进牙髓再生的效果。ZHANG等[32]研究了上皮根鞘细胞来源的外泌体,发现其促进了牙乳头细胞的成牙分化和成血管化,最终生成了牙髓样组织。LI等[33]发现,施万细胞来源的外泌体通过激活牙乳头细胞的转化生长因子β/Smad和转化生长因子β/丝裂原活化蛋白激酶信号通路,对牙乳头细胞增殖和促进成骨、成脂和成神经等多向分化方面起到了关键的调控作用。以上的研究结果证实不同细胞来源的外泌体均可以通过调控干细胞的增殖、迁移和分化促进牙髓血管和神经的生成以及牙本质样组织的形成,进而生成了较理想的牙髓牙本质复合体,成为牙髓再生中理想的生物仿生工具。因此,为了具体体现不同细胞来源外泌体在促进再生中的具体作用,对被普遍应用于牙髓再生中的细胞进行了归纳总结,见表1。
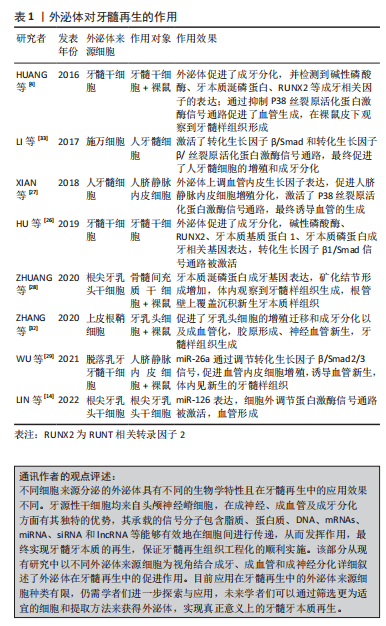
2.2 外泌体的预处理方式 细胞的培养环境高度影响着其分泌外泌体的生物学特性和功能。因此,越来越多的研究聚焦于通过改变外泌体来源细胞的培养环境以改善外泌体的生物学特性,从而增强组织的修复和再生能力。目前改善外泌体功能特性的预处理方式包括低氧诱导、炎性刺激、条件培养基、三维培养、母细胞遗传修饰、超声辐射、机械刺激和药物刺激等方法。
2.2.1 炎性刺激 研究表明,适度炎症条件下可以促进外泌体的分泌量增多[34],从而更好的促进间充质干细胞的增殖与分化,并且发现其可以通过激活相关分子通路进一步增强血管的生成[35];同时由于外泌体分泌量增多等因素,其抗细胞凋亡和保护神经的能力显著增强[36]。研究发现,加入肿瘤坏死因子α后的脐带间充质干细胞分泌的外泌体相较于常规培养下衍生的外泌体在管腔形成能力方面具有更好的促进效果,瘢痕的堆积明显减少,皮肤缺损创面的修复愈合明显加快[15,37]。相似地,在肿瘤坏死因子α诱导的炎症环境下,脐带间充质干细胞分泌的外泌体有效促进牙周膜干细胞的增殖和迁移,且在研究中发现其效果呈浓度依赖性上升的趋势,这为牙周组织的再生提供了新思路[38]。脂多糖属于革兰阴性菌的细胞壁成分,也是目前应用较多的炎性刺激方式。在一项有关脂多糖刺激下根尖牙乳头干细胞衍生的外泌体抗凋亡的研究中,发现高浓度脂多糖刺激下衍生的外泌体被邻近低浓度细胞摄取,减轻了细胞凋亡发生率,促使更多低浓度炎症环境下的细胞存活[15]。
炎症刺激目前作为研究较多的一种细胞刺激方式,可以有效促进外泌体对组织修复再生的作用,上述研究结果表明炎性刺激预处理方式可使外泌体的分泌量增加且增强其生物活性,其衍生的外泌体对细胞的增殖迁移和定向分化以及抗凋亡等具有调控作用,且在血管生成和牙周再生等方面,其增强效果可能与外泌体的miRNA等小分子的差异表达有一定关系,具体机制需要进一步的探索与研究。
2.2.2 低氧诱导 大量研究表明低氧环境下会激活低氧诱导因子1α,其对外泌体及多种生长因子具有较强的生物调节作用,可以增加细胞间的通讯且促进外泌体的分泌[39-42]。有研究发现,外泌体的促血管生成相关因子miRNA如miR-126,miR-130a和miR-210在低氧条件下表达显著上调,在此过程中低氧诱导因子1α被激活,血管内皮生长因子、表皮生长因子和成纤维细胞生长因子等同时表达并且上调,共同促进了细胞的增殖与迁移且促进血管的生成[43-45],对血管损伤具有一定的保护作用。此外,经低氧诱导衍生的外泌体也较常态培养下对促成骨分化的效果更显著。LIU等[44]研究发现,间充质干细胞经过低氧诱导后,新骨加速形成,发现其中低氧诱导因子1α 被激活并介导外泌体miR-126的产生,通过miR-126和SPRED1/Ras/ERK信号通路并且加速了成骨分化。研究发现不同氧浓度诱导下衍生的外泌体对组织再生与修复也具有不同作用。与常氧(体积分数21% O2)条件相比,ALMERIA等[46]研究发现,在一般低氧(体积分数5% O2)条件下培养的脂肪间充质干细胞来源外泌体,可以显著诱导血管形成;在中度低氧(体积分数1% O2)条件下培养的心肌细胞分泌的外泌体,更加有效地促进了血管的生成,还可以触发抗凋亡、免疫调节以及抗氧化应激等反应[43,47];另一项研究报道,在严重低氧(体积分数0% O2)以致无氧条件下,细胞在培养12 h后分泌的外泌体仍具有生物活性,可以呈剂量依赖式促进血管生成[48],对其作用机制进行探讨,推测可能为低氧条件引起糖酵解增加,乳酸堆积,导致细胞外环境变为酸性微环境,而酸性微环境中外泌体的分泌量可增加,其含有的蛋白质及RNA等受到环境中pH值的影响,功能特性发生了一定改变的
缘故。
因此,细胞在低氧下的培养时间和具体氧浓度均会对外泌体的生物学特性有一定的影响,低氧条件下低氧诱导因子1α、血管内皮生长因子和表皮生长因子等作用于管腔形成和血管功能调节等方面发挥了重要的协同作用,但在不同领域中的具体最适宜的作用浓度与时间有待进一步研究论证。
2.2.3 条件培养基 不同培养条件下细胞分泌的外泌体其生物学特性是不同的。细胞培养基中的不同成分不仅对干细胞的生物活性状态起决定性作用,而且对其分泌的外泌体功能性质也具有显著影响。细胞培养过程中某些成分的缺乏会影响外泌体的功能性质,例如培养心肌细胞时培养基去除葡萄糖成分,会促进外泌体对血管的生成作用[49];而在培养基中去除癌细胞的关键代谢底物谷氨酰胺则导致癌细胞外泌体分泌增加,促进肿瘤细胞的增殖和更新,并在异体移植小鼠模型体内参与调节血管生成[50]。血清作为体外实验培养的必须营养构成组分,对于细胞生命活动的维持必不可缺。然而,干细胞在培养过程中使用血清,存在基于干细胞治疗的临床转化风险,例如有害的异种免疫反应,无论是通过内化和随后的抗原呈递,还是通过附着在细胞表面的方式,都存在移植后成为抗原底物引起免疫排斥反应等效应风险,虽然这些风险与涉及实际治疗的细胞有关,但它们也适用于衍生的外泌体,因为外泌体的膜反映了母细胞的膜[51], 因此,也可能呈现这些抗原物质。上述研究表明在不同研究中通过在细胞培养基中添加/减少部分成分可以更精准地满足不同研究需要,以达到改善外泌体生物学特性和组织修复再生效果的目的,这为外泌体的优化提供了另一预处理方式,并为其后续用于再生医学奠定基础。
2.2.4 三维培养 目前,细胞培养存在两种方式,即传统的单层形式二维培养和新兴生物学方式仿生三维培养。细胞的三维培养能够更好地模拟体内微环境,并被证明在增强其治疗特性方面对间充质干细胞有益[52],其中以形成球状结构的三维培养可以更好地模拟体内细胞生理环境,从而影响其行为和基因表达[53]。据研究报道,与二维培养的间充质干细胞相比,干细胞的三维培养可增强其潜在治疗效果,例如在免疫调节、血管生成、抗纤维化和分化潜力方面的效力[54],且由于细胞相互接触,三维培养中的细胞具有更多激活的信号通路,这导致以旁分泌方式分泌的外泌体或各种生长因子的释放,触发相应的生物学效应[55]。研究表明,三维培养脐带间充质干细胞衍生的外泌体一方面显示出增强的促成纤维细胞迁移和增殖的能力,加速伤口的愈合[56],另一方面也可以显著抑制成纤维细胞活化,减少肺纤维化发生[57]。YAN等[58]研究脐带间充质在常规二维培养下产生的外泌体和三维培养下产生的外泌体在中空纤维生物反应器中用于治疗软骨修复的干细胞细胞过程和机制中发现,三维培养模式下外泌体的产量比二维高7.5倍,通过激活转化生长因子β/Smad2/3信号通路,三维培养下衍生的外泌体显著刺激了软骨细胞增殖、迁移和基质合成,抑制了细胞凋亡。证实细胞的三维培养模式显示出比二维更好的治疗效果,为组织的修复再生提供了新思路。
2.2.5 其他预处理方式 除了上述提及的预处理方式外,对外泌体的预处理方式还有机械刺激和辐射刺激、基因遗传修饰、多种细胞因子、药物、化学刺激、激素、乙醇、氧化应激及热应激等[4,17,46,59-63]。朱梦远[64]研究发现,通过低强度脉冲超声作用分泌的外泌体,对牙囊干细胞的生物学作用具有显著影响,增强其增殖与成骨分化,促进牙周再生;紫外线B是一种物理辐射,对人体存在一定影响,特别在皮肤方面,过度暴露于紫外线B会导致晒伤和皮肤癌,而适当的紫外线B照射可以调节免疫系统等。有研究表明,紫外线B辐照可以促进外泌体释放,并以剂量和时间依赖性方式促进糖尿病角质形成细胞分泌外泌体;以干预24 h,100 和 1 800 J/m2的剂量效果最为突出[65],被选为有效的低通量和高通量剂量,低通量紫外线B照射角质形成细胞衍生的外泌体提高了施万细胞的活力,而来自高通量照射的外泌体则抑制了施万细胞的活力。
LI等[60]研究表明,转染低氧诱导因子1α的骨髓间充质干细胞分泌的外泌体在体外表现出更强的促成骨和促血管生成作用;在兔体内的激素性股骨头缺血坏死模型中,将富含低氧诱导因子1α的外泌体注射到股骨头坏死区,发现骨再生和血管生成加快。有研究也证实了药物预处理间充质干细胞,如阿托伐他汀及二甲基甘氨酸等,其分泌的外泌体可上调细胞活性,通过促进血管内皮生长因子的释放、靶向调节蛋白激酶B/哺乳动物雷帕霉素靶蛋白等相关信号通路与生长因子增强血管生成与骨再生[61,66]。此外,有研究发现,褪黑素预处理间充质干细胞,可以调动细胞活性,提高细胞存活和植入能力,包括促进血管内皮生长因子表达、新生血管增加以及改善神经行为,促进体内神经再生等[67];而经褪黑素预处理分泌的外泌体不仅可以改善糖尿病创口的修复愈合[68],还可以加强间充质干细胞的成骨分化,促进骨再生,有效治疗骨质疏松等疾病[69]。
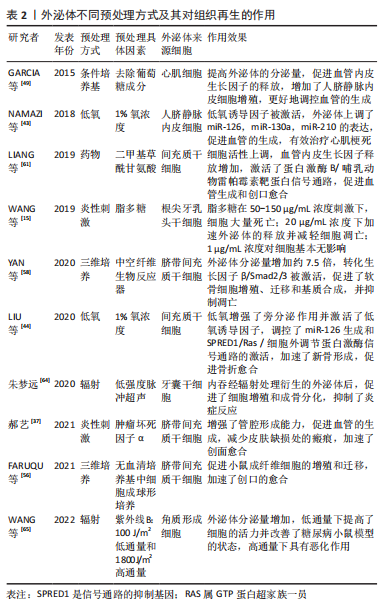
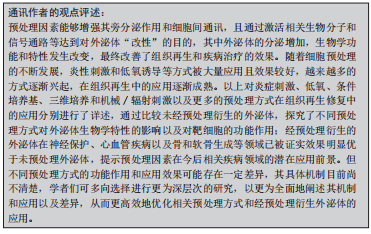
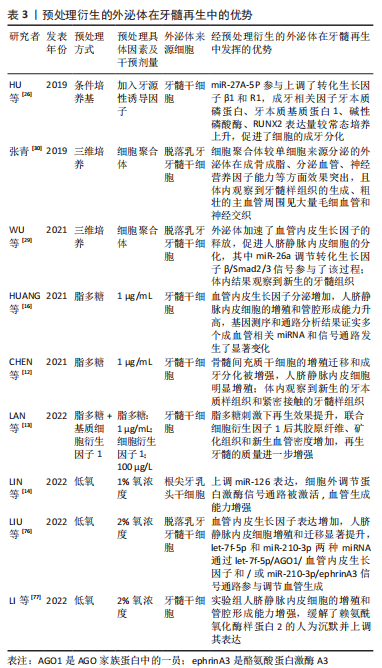
GURUNATHAN等[70]研究表明,氧化应激也可促使细胞外泌体分泌增加,在组织再生领域具有良好的应用潜力。因此,一个有利的培养微环境是干细胞生存及发挥作用的必要条件,而经过对细胞进行预处理分泌的外泌体,展现出了良好生物学效应,预处理因素能够通过激活相关生物活性因子、miRNA 或信号通路等来增加外泌体的释放量,且增强了细胞衍生的外泌体生物学活性,进而改善作用效果,提示其在组织再生领域的良好应用前景。文章总结了不同预处理方式以及经其衍生的外泌体对组织修复再生等的作用,见表2,图3。

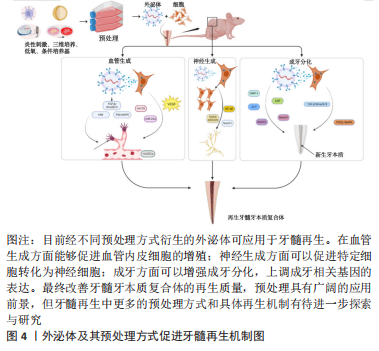
2.3 外泌体的预处理方式对牙髓再生的作用 牙髓再生是一个涉及牙髓血管、神经以及牙髓组织和牙本质生成的复杂过程,其新生的复合组织被称为牙髓牙本质复合体。在该过程中外泌体能够有效诱导血管内皮细胞新生进而促进牙髓血管的生成和诱导特定细胞分化为神经细胞,两者的生成相互交织,且外泌体可通过促进细胞的成牙分化诱导新生牙本质的沉积。研究表明,预处理因素能够有效改善牙髓再生的效果,但不同预处理方式衍生的外泌体在牙髓再生中的具体作用效果并不完全相同。
2.3.1 炎性刺激 相较于常规培养条件下细胞分泌的外泌体而言,经炎性刺激预处理衍生的外泌体在牙髓再生中具明显的改善作用。有学者发现,脂多糖刺激可以显著增加牙囊细胞衍生外泌体的分泌,其比常态培养具有更强的增殖、迁移和成牙分化能力[71];LI等[17]探索了经脂多糖刺激下牙髓干细胞衍生的外泌体对施万细胞的作用,与常规分泌的外泌体相比,炎性刺激组可以更好的促进施万细胞的增殖和迁移,并且可以促进其成牙分化。推测其机制可能由于炎性刺激增强了旁分泌作用进而增加了细胞间通讯,且由于外泌体分泌量增多,强化了对细胞的增殖分化等作用。然而,炎性刺激发挥作用时也与其刺激浓度具有相关性。有研究表明,与常态培养相比,当牙髓干细胞经1 μg/mL 脂多糖刺激分泌的外泌体与施万细胞共培养时,施万细胞显示出更好的迁移和成牙分化能力;5 μg/mL 脂多糖刺激牙髓干细胞所分泌的外泌体,提高了人脐静脉内皮细胞的增殖,更好地诱导血管的形成[16-17]。但并非浓度越高其作用效果越好,在一项关于炎症状态下外泌体对根尖牙乳头干细胞抗凋亡的研究中发现高浓度(50-150 μg/mL)脂多糖刺激将导致细胞大量死亡,而20 μg/mL 脂多糖刺激下分泌的外泌体最多,且对细胞的凋亡有较好的抑制作用[15],提示在相关研究中,选取适宜的炎症刺激浓度或将十分重要。上述研究表明,经脂多糖刺激衍生的外泌体其分泌量得到了增加,且可以有效促进细胞的增殖并增强成牙分化能力。
脂肪间充质干细胞已被证明可以有效促进牙髓再生[72]。WU等[35]研究了脂肪间充质干细胞经脂多糖刺激衍生的外泌体对人脐静脉内皮细胞的作用,结果发现通过激活核转录因子κB信号通路,增强了人脐静脉内皮细胞的迁移和血管形成能力;在另一项研究中,脂多糖刺激牙髓干细胞衍生的外泌体,通过检测其促血管生成能力,结果发现相较于常态培养,炎性刺激组增加了血管内皮生长因子的释放,提高了人脐静脉内皮细胞的增殖和迁移,并通过基因测序分析表明,炎症组中含有多个差异化表达基因和多条血管生成相关通路[16],具有广泛而确切的血管生成潜能,上述研究证实了在脂多糖刺激下,外泌体可以加速血管内皮生长因子的释放,进一步促进了人脐静脉内皮细胞的增殖和管腔形成的能力,进而诱导了牙髓血管的生成,表明经预处理衍生的外泌体在新生血管领域具有广阔的应用前景。此外,在牙髓再生中,牙髓牙本质复合体的形成是关键,研究已证实外泌体可以促进牙髓样组织生成[4,28-31]。
最近CHEN等[12]在研究中发现,经脂多糖刺激下牙髓干细胞衍生的外泌体在体外增强了骨髓间充质干细胞的增殖迁移与成牙分化,且高表达血管内皮生长因子和神经束蛋白。表明在脂多糖刺激下,外泌体通过自身的“改变”,影响了人脐静脉内皮细胞的增殖和成牙分化等,且较常态培养下效果更佳。在后期的动物研究中观察到小鼠体内新生了血管组织,胶原形成及新生矿化组织规则排列在原有根管壁上,并且形成了类牙髓样组织,其再生组织的质量有显著提高。在一项最新的研究中,学者们比较了经脂多糖刺激下牙髓干细胞衍生的外泌体和常态培养下分泌的外泌体对牙髓再生的促进效果[13],体内结果观察到炎症组新生的牙髓样组织和血管数量明显较多,当同时联合基质细胞衍生因子1时,炎症组的作用再次增强,其矿化组织更规则,胶原纤维排列更加整齐,显示出较强的成牙髓能力。细胞经炎性刺激后衍生的外泌体对成神经、成血管和成牙分化具有增强作用,该方式具有潜在的应用前景,但其具体浓度影响着最终的作用效果,如何对其进行精准调控决定了今后的发展,为未来在牙髓再生领域提高再生质量提供了进一步方向。
2.3.2 低氧诱导 有研究发现,低氧条件下细胞分泌的外泌体可以更好的促进血管形成[73-74],具体体现在,低氧诱导来源的外泌体可促使血管生成相关miRNA和血管内皮生长因子、表皮生长因子、成纤维细胞生长因子等及其受体(血管内皮生长因子 R2,R3)表达增多,并通过调节相关信号通路,显著促进血管内皮细胞的增殖、迁移和血管生成[75]。因此,用含有编码促血管生成因子的基因DNA质粒转染或在分泌外泌体的细胞中引入蛋白形式的促血管生成因子能够使外泌体的血管生成潜力增加。
LIN等[14]探索了经低氧诱导下根尖牙乳头干细胞衍生的外泌体对人脐静脉内皮细胞血管生成的影响,结果证实其血管生成能力较常氧组增强,最终更好地促进了血管形成。其中细胞外调节蛋白激酶信号通路被激活,miR-126表达上调,而miR-126表达与血管形成相关[43]。在另一项研究中,LIU等[76]探索了低氧诱导脱落乳牙牙髓干细胞细胞分泌的外泌体在血管生成中的潜在机制,相较于常氧条件,低氧组显著促进了血管内皮生长因子表达增加以及人脐静脉内皮细胞增殖和迁移,同时进行了miRNA测序,发现低氧组同时转运let-7f-5p和miR-210-3p两种miRNA通过let-7f-5p/AGO1/血管内皮生长因子和/或miR-210-3p/ephrinA3信号通路参与调节血管生成。而在另一项研究中,通过对比常氧条件下细胞分泌的外泌体,发现低氧诱导下牙髓干细胞衍生的外泌体更有效促进了人脐静脉内皮细胞的增殖、迁移和血管生成能力。赖氨酰氧化酶样2能够参与多种功能,如血管保护,血管新生和组织修复等,在两组中同时沉默了赖氨酰氧化酶样2后,发现低氧组缓解了赖氨酰氧化酶样2同时介导了血管的生成[77]。血管的生成是牙髓再生整个过程中十分重要的步骤,上述研究从分子、蛋白等角度进一步证明了经低氧诱导衍生的外泌体在血管生成方面效果显著,其中缺氧诱导因子1α以及相关促血管生成因子的协同作用增加了细胞间的通讯以及旁分泌作用,这为今后进行牙髓再生奠定了前期体外研究基础。
2.3.3 其他预处理方式 通过调整细胞培养基成分进而影响外泌体的生物学特性与效果已被证实,目前在牙髓再生领域中已涉及相关研究。有学者在细胞培养基中加入牙源性诱导因子,与常态培养条件相比,发现牙源性诱导条件下分泌的牙髓干细胞外泌体能够更好的诱导干细胞成牙分化。最终在体内结果中发现类牙髓样组织的新生,新生的牙本质沉积更加均匀[4,26]。在另一项相似的研究中,学者们通过RNA测序探索了在牙源性诱导条件下牙髓干细胞成牙分化的相关机制,其中实验组的成牙相关因子牙本质磷蛋白、牙本质基质蛋白1、碱性磷酸酶、RUNX2表达较未经处理的外泌体对照组上调明显,其中miR-27A-5P通过转化生长因子β1促进了成牙分化[4,26]。在牙源性诱导条件下产生的外泌体分别在现象和机制方面被证明其成牙分化的有效性,在组织再生等领域中该预处理方式也同样有效,该方式的转化应用效果值得肯定,这为研究者们在今后将更多的预处理方式转化应用在牙髓再生领域中提供了新的思路和策略。
三维培养主要通过生物材料或形成球形聚集体空间,模拟重现细胞的体内生理环境,以此实现避免细胞的过渡性损伤,最大化恢复细胞的生物学功能。生物支架材料不仅可以为外泌体提供功能性附着搭载,其三维空间也为外泌体发挥作用提供了功能性保障。细胞聚合体也是另外一种形式的三维培养方式,单细胞培养来源的外泌体已被证实可以促进牙髓再生,那么处于聚合体状态和处于单细胞状态下所分泌的外泌体是否存在差异以及聚合体来源的外泌体牙髓再生效果是否更佳?有学者研究发现,脱落乳牙牙髓干细胞聚合体来源的外泌体比单细胞状态促血管神经修复再生的miRNA表达增多,并促进脱落乳牙牙髓干细胞分泌多种血管神经营养因子以及多向谱系分化,在原位移植和异位移植体内实验中,还观察到新生的牙髓样组织及其神经血管组织的再生质量有较好提升[29-30]。同时,在FARUQU等[78]的最新研究中,观察到3D培养环境中的牙髓多能样干细胞能够形成球状体,随着培养时间的延长而逐渐减小,表明它们正在缓慢地经历着细胞凋亡,但仍保持其完整性和未分化状态,相较二维环境,三维培养下细胞的多能性进一步提升,进一步推测其可能具有定向分化能力。该研究还首次报告了从牙髓多能样干细胞的三维培养中分离出外泌体,其分泌量远远高于二维培养环境下衍生的外泌体量,证明了三维培养环境是一个有效且值得深入探究的预处理方式。
与常态培养细胞分泌的外泌体相比较,经预处理衍生的外泌体功能特性有显著改善,有效促进了牙髓再生,或将成为一种提高牙髓再生作用效果的有效方式。其中炎性刺激可以提高成牙分化和血管生成的效果,最终的再生牙髓质量有了较好的提升;低氧诱导则可以产生相关的因子如低氧诱导因子等促进血管的新生,在血管生成方面具有较好的效果,而充足的血运是保证牙髓再生的重要前提基础;牙本质的生成同样重要,条件培养基中添加了成牙诱导因子则更有效地促进了成牙分化,为牙髓再生提供了更加适宜的管内微环境。然而,目前经预处理衍生的外泌体用于牙髓再生的研究尚不多见,有待进一步的研究和探索;且各预处理因素的具体作用机制如何;预处理因素间结合应用效果怎样;是否存在不良反应以及如何选取最佳预处理方法和探索更多方法等仍需进一步探索研究。为了具体体现不同的预处理方式和同一预处理方式下不同的具体因素对牙髓再生的作用,文章对经预处理衍生的外泌体进行了总结归纳,见表3,图4。

| [1] GANESH V, SEOL D, GOMEZ-CONTRERAS PC, et al. Exosome-based cell homing and angiogenic differentiation for dental pulp regeneration. Int J Mol Sci. 2022;24(1):466. [2] IVICA A, ZEHNDER M, WEBER FE. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in regenerative endodontics. Eur Cell Mater. 2021;41:233-244. [3] TANNOURY CA, AN HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14(3):552-559. [4] HUANG CC, NARAYANAN R, ALAPATI S, et al. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-115. [5] AZOIDIS I, COX SC, DAVIES OG. The role of extracellular vesicles in biomineralisation: current perspective and application in regenerative medicine. J Tissue Eng. 2018;9:1544427758. [6] LIU M, SUN Y, ZHANG Q. Emerging role of extracellular vesicles in bone remodeling. J Dent Res. 2018;97(8):859-868. [7] VAN NIEL G, D’ANGELO G, RAPOSO G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213-228. [8] SIMPSON RJ, JENSEN SS, LIM JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083-4099. [9] YU S, CHEN H, GAO B. Potential therapeutic effects of exosomes in regenerative endodontics. Arch Oral Biol. 2020;120:104946. [10] 艾晓青,窦磊.外泌体优化策略的研究进展[J].临床医学研究与实践, 2021,6(16):190-192. [11] 刘威.预处理的外泌体对骨质疏松骨整合及糖尿病创面修复作用的研究[D].上海:中国人民解放军海军军医大学,2021. [12] CHEN WJ, XIE J, LIN X, et al. The role of small extracellular vesicles derived from lipopolysaccharide-preconditioned human dental pulp stem cells in dental pulp regeneration. J Endod. 2021;47(6):961-969. [13] LAN BY, LIN X, CHEN WJ, et al. Effect of lipopolysaccharide-stimulated exosomes from human dental pulp stem cells combined with stromal cell-derived factor-1 on dental pulp regeneration. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022;57(1):60-67. [14] LIN X, WANG H, WU T, et al. Exosomes derived from stem cells from apical papilla promote angiogenesis via miR-126 under hypoxia. Oral Dis. 2022. doi: 10.1111/odi.14285. [15] WANG HS, YANG FH, WANG YJ, et al. Odontoblastic exosomes attenuate apoptosis in neighboring cells. J Dent Res. 2019;98(11):1271-1278. [16] HUANG X, QIU W, PAN Y, et al. Exosomes from LPS-stimulated hDPSCs activated the angiogenic potential of HUVECs in vitro. Stem Cells Int. 2021; 2021:6685307. [17] LI J, JU Y, LIU S, et al. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2021;62(3):277-286. [18] CHEN Y, YANG XT, MA Y, et al. Exosomes-based strategies for dental pulp regeneration. Zhonghua Kou Qiang Yi Xue Za Zhi. 2021;56(7):709-714. [19] MAI Z, CHEN H, YE Y, et al. Translational and clinical applications of dental stem cell-derived exosomes. Front Genet. 2021;12:750990. [20] OGOREVC E, KRALJ-IGLIC V, VERANIC P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013;47(3):197-205. [21] ANA ID, BARLIAN A, HIDAJAH AC, et al. Challenges and strategy in treatment with exosomes for cell-free-based tissue engineering in dentistry. Future Sci OA. 2021;7(10):FSO751. [22] HUA S, BARTOLD PM, GULATI K, et al. Periodontal and dental pulp cell-derived small extracellular vesicles: a review of the current status. Nanomaterials (Basel). 2021;11(7):1858. [23] RAMASUBRAMANIAN L, KUMAR P, WANG A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules. 2019;10(1):48. [24] JIANG N, XIANG L, HE L, et al. Exosomes mediate epithelium-mesenchyme crosstalk in organ development. ACS Nano. 2017;11(8):7736-7746. [25] MAO Q, NGUYEN PD, SHANTI RM, et al. Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng Part A. 2019;25(11-12):887-900. [26] HU X, ZHONG Y, KONG Y, et al. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFbeta1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther. 2019;10(1):170. [27] XIAN X, GONG Q, LI C, et al. Exosomes with highly angiogenic potential for possible use in pulp regeneration. J Endod. 2018;44(5):751-758. [28] ZHUANG X, JI L, JIANG H, et al. Exosomes derived from stem cells from the apical papilla promote dentine-pulp complex regeneration by inducing specific dentinogenesis. Stem Cells Int. 2020;2020:5816723. [29] WU M, LIU X, LI Z, et al. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-beta/SMAD2/3 signalling. Cell Prolif. 2021;54(7):e13074. [30] 张青. 乳牙牙髓干细胞聚合体来源外泌体在牙髓再生中的作用研究[D].西安:中国人民解放军空军军医大学,2019. [31] 乔新. 负载外泌体的纤维蛋白/明胶支架促进牙髓再生的初步研究[D].重庆:重庆医科大学,2021. [32] ZHANG S, YANG Y, JIA S, et al. Exosome-like vesicles derived from Hertwig’s epithelial root sheath cells promote the regeneration of dentin-pulp tissue. Theranostics. 2020;10(13):5914-5931. [33] LI Z, LIANG Y, PAN K, et al. Schwann cells secrete extracellular vesicles to promote and maintain the proliferation and multipotency of hDPCs. Cell Prolif. 2017;50(4):e12353. [34] PIZZATTO LN, MENESES C, DINIZ EA, et al. Angiotensin ii regulates proliferation and function of stem cells of apical papilla. J Endod. 2020;46(6):810-817. [35] WU SC, KUO PJ, RAU CS, et al. Increased angiogenesis by exosomes secreted by adipose-derived stem cells upon lipopolysaccharide stimulation. Int J Mol Sci. 2021;22(16):8877. [36] ZHENG Y, HE R, WANG P, et al. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037-2049. [37] 郝艺.TNF-α诱导的脐带间充质干细胞外泌体对修复细胞及创面愈合的影响研究[D].遵义:遵义医科大学,2021. [38] 田萧羽,杨烁,朱彪,等.炎症微环境中脐带干细胞外泌体对牙周膜干细胞增殖和迁移的影响[J].解放军医学院学报,2021,42(5):541-547. [39] ZHANG W, ZHOU X, YAO Q, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am J Physiol Renal Physiol. 2017,313(4):F906-F913. [40] DORAYAPPAN K, WANNER R, WALLBILLICH JJ, et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806-3821. [41] YU Y, MIN Z, ZHOU Z, et al. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp Cell Res. 2019;385(1):111649. [42] KUMAR A, DEEP G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23-30. [43] NAMAZI H, MOHIT E, NAMAZI I, et al. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J Cell Biochem. 2018;119(5):4150-4160. [44] LIU W, LI L, RONG Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [45] HAN Y, REN J, BAI Y, et al. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59-68. [46] ALMERIA C, WEISS R, ROY M, et al. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol. 2019;7:292. [47] COLLINO F, LOPES JA, CORREA S, et al. Adipose-derived mesenchymal stromal cells under hypoxia: changes in extracellular vesicles secretion and improvement of renal recovery after ischemic injury. Cell Physiol Biochem. 2019;52(6):1463-1483. [48] GRAY WD, FRENCH KM, GHOSH-CHOUDHARY S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2): 255-263. [49] GARCIA NA, ONTORIA-OVIEDO I, GONZALEZ-KING H, et al. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10(9):e138849. [50] FAN SJ, KROEGER B, MARIE PP, et al. Glutamine deprivation alters the origin and function of cancer cell exosomes. EMBO J. 2020;39(16):e103009. [51] COLOMBO M, RAPOSO G, THERY C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [52] YIN F, WANG WY, JIANG WH. Human umbilical cord mesenchymal stem cells ameliorate liver fibrosis in vitro and in vivo: from biological characteristics to therapeutic mechanisms. World J Stem Cells. 2019;11(8):548-564. [53] PETRENKO Y, SYKOVA E, KUBINOVA S. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8(1):94. [54] CESARZ Z, TAMAMA K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016;2016:9176357. [55] ZHANG J, WANG M, CHA JM, et al. The incorporation of 70s bioactive glass to the osteogenic differentiation of murine embryonic stem cells in 3D bioreactors. J Tissue Eng Regen Med. 2009;3(1):63-71. [56] FARUQU FN, LIAM-OR R, ZHOU S, et al. Defined serum-free three-dimensional culture of umbilical cord-derived mesenchymal stem cells yields exosomes that promote fibroblast proliferation and migration in vitro. FASEB J. 2021;35(1):e21206. [57] XU C, HOU L, ZHAO J, et al. Exosomal let-7i-5p from three-dimensional cultured human umbilical cord mesenchymal stem cells inhibits fibroblast activation in silicosis through targeting TGFBR1. Ecotoxicol Environ Saf. 2022;233:113302. [58] YAN L, WU X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36(2):165-178. [59] YAN L, LIU G, WU X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J Orthop Translat. 2021;26:111-120. [60] LI H, LIU D, LI C, et al. Exosomes secreted from mutant-HIF-1alpha-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int. 2017;41(12):1379-1390. [61] LIANG B, LIANG JM, DING JN, et al. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10(1):335. [62] JAFARI R, RAHBARGHAZI R, AHMADI M, et al. Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J Transl Med. 2020;18(1):474. [63] AWOYEMI AA, BORCHERS C, LIU L, et al. Acute ethanol exposure stimulates microvesicle particle generation in keratinocytes. Toxicol Lett. 2022;355: 100-105. [64] 朱梦远.LIPUS诱导的hDFSCs外泌体对hDFSCs增殖分化的作用机制研究[D].重庆:重庆医科大学,2020. [65] WANG J, POTHANA K, CHEN S, et al. Ultraviolet B irradiation alters the level and mir contents of exosomes released by keratinocytes in diabetic condition. Photochem Photobiol. 2022;98(5):1122-1130. [66] HUANG P, WANG L, LI Q, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353-367. [67] TANG Y, CAI B, YUAN F, et al. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant. 2014;23(10):1279-1291. [68] LIU W, YU M, XIE D, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259. [69] 申恩谱,黄霸,刘丹平,等.褪黑素预处理的骨髓间充质干细胞外泌体促进骨髓间充质干细胞成骨[J].中国组织工程研究,2022,26(30):4800-4805. [70] GURUNATHAN S, KANG MH, JEYARAJ M, et al. Palladium nanoparticle-induced oxidative stress, endoplasmic reticulum stress, apoptosis, and immunomodulation enhance the biogenesis and release of exosome in human leukemia monocytic cells (THP-1). Int J Nanomedicine. 2021;16: 2849-2877. [71] SHI W, GUO S, LIU L, et al. Small extracellular vesicles from lipopolysaccharide-preconditioned dental follicle cells promote periodontal regeneration in an inflammatory microenvironment. ACS Biomater Sci Eng. 2020;6(10):5797-5810. [72] COSTELA-RUIZ VJ, MELGUIZO-RODRIGUEZ L, BELLOTTI C, et al. Different sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. 2022;23(11):6356. [73] FAN G C. Hypoxic exosomes promote angiogenesis. Blood. 2014;124(25): 3669-3670. [74] GONZALEZ-KING H, GARCIA NA, ONTORIA-OVIEDO I, et al. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35(7):1747-1759. [75] HAN Y, REN J, BAI Y, et al. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59-68. [76] LIU P, QIN L, LIU C, et al. Exosomes derived from hypoxia-conditioned stem cells of human deciduous exfoliated teeth enhance angiogenesis via the transfer of let-7f-5p and miR-210-3p. Front Cell Dev Biol. 2022;10:879877. [77] LI B, XIAN X, LIN X, et al. Hypoxia alters the proteome profile and enhances the angiogenic potential of dental pulp stem cell-derived exosomes. Biomolecules. 2022,12(4):575. [78] FARUQU FN, ZHOU S, SAMI N, et al. Three-dimensional culture of dental pulp pluripotent-like stem cells (DPPSCs) enhances Nanog expression and provides a serum-free condition for exosome isolation. FASEB Bioadv. 2020; 2(7):419-433. |
| [1] | 刘瀚峰, 王晶晶, 余云生. 人造外泌体治疗心肌梗死:应用现状及前景[J]. 中国组织工程研究, 2024, 28(7): 1118-1123. |
| [2] | 马树微, 何 生, 韩 冰, 张缭云. 间充质干细胞来源外泌体治疗动物急性肝衰竭的Meta分析[J]. 中国组织工程研究, 2024, 28(7): 1137-1142. |
| [3] | 沈子青, 夏 天, 单一波, 朱睿君, 万昊鑫, 丁 浩, 潘 枢, 赵 军. 负载外泌体水凝胶修饰3D打印支架构建血管化的气道替代物[J]. 中国组织工程研究, 2024, 28(5): 697-705. |
| [4] | 戴 京, 刘沙沙, 沈明敬. 负载外泌体的可注射水凝胶修复种植体周围骨缺损[J]. 中国组织工程研究, 2024, 28(3): 347-354. |
| [5] | 杨启航, 蒲 锐, 陈子扬, 冷思逸, 宋永晶, 刘 辉, 杜光友. 肠道菌群代谢物在肥胖调控中的作用与机制[J]. 中国组织工程研究, 2024, 28(2): 308-314. |
| [6] | 龙 宜, 杨佳明, 叶 花, 钟燕彪, 王茂源. 细胞外囊泡在少肌性肥胖中的作用及机制[J]. 中国组织工程研究, 2024, 28(2): 315-320. |
| [7] | 李成明, 薛冬令, 杨鑫宇, 肖 驰, 崔大平. 活血化瘀中药联合富血小板血浆改善激素性股骨头坏死的作用机制[J]. 中国组织工程研究, 2024, 28(2): 288-294. |
| [8] | 史东子, 张 华, 孟 昶, 李昕睿, 董盼盼, 田雪文, 王清路. 时钟基因调控低氧训练肥胖大鼠白色脂肪组织棕色化[J]. 中国组织工程研究, 2024, 28(16): 2473-2480. |
| [9] | 刘 云, 靳嘉岩, 刘玉斌, 李 强, 任博媛, 吴祖泽, 周钢桥, 靳继德. 丙二醇联合肝细胞生长因子修饰牙髓干细胞外泌体保护人表皮细胞的放射损伤[J]. 中国组织工程研究, 2024, 28(13): 2002-2008. |
| [10] | 郑 颖, 黄珂敏. 外泌体研究态势和前瞻的计量与可视化分析[J]. 中国组织工程研究, 2024, 28(13): 2126-2132. |
| [11] | 饶 瑾, 姜 水, 石海山. 基于炎症微环境调控组织工程再生性牙髓治疗[J]. 中国组织工程研究, 2024, 28(10): 1620-1625. |
| [12] | 陈冠廷, 张琳琪, 李清茹. 外泌体在慢性肾脏病诊疗中的研究热点与趋势[J]. 中国组织工程研究, 2024, 28(1): 86-92. |
| [13] | 黄勇彬, 王 涛, 娄园一, 庞景群, 陈光华. 间充质干细胞促进肌肉组织修复的应用前景[J]. 中国组织工程研究, 2024, 28(1): 107-112. |
| [14] | 马岁录, 何志军, 刘 涛, 李 岩, 何元旭, 何 波, 王威威, 魏晓涛. 中药单体调控“细胞自噬”防治皮瓣坏死[J]. 中国组织工程研究, 2024, 28(1): 153-158. |
| [15] | 王宪峰, 王 锟, 孙 晗, 孙晓亮, 言力韬. 脐带间充质干细胞外泌体LncRNA H19修复软骨损伤的机制[J]. 中国组织工程研究, 2024, 28(1): 20-25. |
外泌体是体内活细胞分泌的一种膜性小囊泡,属于胞外囊泡的一种,可介导细胞相互间信息的传递[5]。人们根据胞外囊泡的大小、形态以及生物特征将其分为外泌体、微囊泡以及凋亡小体[6]。外泌体在核内体网中形成,当多囊泡小体与胞膜融合时释放出细胞,直径50-150 nm。胞外囊泡以胞膜出芽的方式产生,仅包括局部的细胞溶质蛋白和核酸,直径在50-1 000 nm。凋亡小体是死亡细胞的碎片,以核碎裂形成的微粒形式存在,直径在500-2 000 nm[7]。外泌体比其他囊泡生物学特性清晰明确,化学性质更稳定,组织选择性高,能高效修复受损组织,易于保存方便运输[2]。研究发现不同细胞来源的外泌体具有不一样的功能,并且它们承载的信号分子包含脂质、蛋白质、DNA、mRNAs、miRNA、siRNA和lncRNA和它们所存在的微环境紧密相关联[8]。通过配体/受体识别,大胞饮、内吞、吞噬或直接与受体细胞胞膜融合等方式,外泌体进入到受体细胞内,并将携带的信号分子释放到受体细胞中,从而触发了受体细胞或器官中必要的分子反应。可以调节间充质干细胞的增殖、迁移和定向分化;同时可以促进干细胞的成牙、成神经和成血管分化,诱导牙髓组织再生[5,9]。
近期研究表明,相比于常规培养条件下细胞产生的外泌体,经预处理的细胞分泌的外泌体其生物学作用显著增强,且不同预处理方式产生的外泌体其生物学特性及作用效果存在明显差异[10-11]。目前对外泌体来源细胞的预处理方式主要有培养条件改变、母细胞遗传修饰、超声辐射、机械刺激、药物刺激、低氧预处理和构建炎症微环境(脂多糖刺激)等,而应用在牙髓再生领域的主要有低氧、炎症刺激、条件培养基和三维培养的方式[4,12-14]。研究发现相比于常态培养下细胞分泌的外泌体,来源于预处理细胞分泌的外泌体能更好地促进牙髓组织的再生[12-13,15-17]。目前不同预处理方式衍生的外泌体对牙髓再生的作用已经得到大量关注,但经预处理后细胞衍生的外泌体应用于牙髓再生的相关综述仍不多见。因此,文章将对外泌体及其预处理方式对牙髓再生的作用作一探讨和总结。 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
1.1.1 检索人及检索时间 第一、二作者及通讯作者,在2022年12月进行检索。
1.1.2 检索文献时限 2022年及之前发表的所有相关中英文文章。
1.1.3 检索数据库 PubMed、Web of Science、中国知网和万方数据库。
1.1.4 检索途径 采用主题词、关键词、标题 / 摘要及部分全文检索。
1.1.5 检索词 中文检索词为“外泌体、牙髓再生、预处理方式、炎性刺激、脂多糖、低氧、条件培养基、三维培养、成牙分化、血管再生、神经再生”。英文检索词为“Exosomes, Pulp regeneration, Preconditioning method, Inflammatory stimulation, Lipopolysaccharidesstimulation, Hypoxia, Conditioned medium, Three-dimensional culture, Odontogenic differentiation, Angiogenesis, Neurogenesis”。
1.1.6 检索策略 以PubMed和中国知网数据库检索策略为例,见图1。
1.2 入组标准
1.2.1 纳入标准 ①所述内容为外泌体及其预处理方式促进牙髓再生领域的相关研究和综述;②文章观点具有新意;③论点可靠且具有说服力。1.2.2 排除标准 ①陈旧和重复的文献;②与综述内容相关度不大的文献。
1.3 文献质量评估和数据的提取 在符合要求的231篇文献中,排除与综述内容不相符、陈旧及重复性文章32篇,再次初筛后得到98篇相关文献,阅读所得文献的标题、摘要和结论对文献进行筛选,根据入选标准排除与不相关的实验研究,最终选择保留78篇中英文文献,包括中国知网和万方数据库中文文献8篇,PubMed中英文文献70篇作进一步综述,文献筛选流程见图2。
3.2 作者综述区别于他人他篇的特点 近年来外泌体的供体来源细胞和生物支架种类的选取一直是牙髓再生领域中改善再生效果的研究重点,但这似乎并没有创新式的发展。预处理因素可以有效提高外泌体分泌量,对细胞的增殖、定向分化和抗凋亡以及血管神经的生成等方面均较未经预处理的外泌体的效果有一定提升。目前预处理因素被大量应用在组织修复再生中且效果较好,在牙髓再生领域中预处理也得到了初步的研究和应用,最终的再生牙髓质量有了提高,该方式似乎成为了一种新的能够通过改善外泌体的生物学特性功能进而提高再生牙髓质量的方式。但目前尚未见有关外泌体及其预处理方式应用于牙髓再生的相关综述文章。文章通过介绍外泌体促进牙髓再生的相关机制,总结了应用在组织修复再生等领域的预处理方式和效果,详细叙述了应用在牙髓再生领域中的预处理方式以及经预处理衍生外泌体对牙髓再生的优化作用,从分子、蛋白等角度阐述了经预处理衍生外泌体的优势以及对血管、神经和成牙分化的效果,肯定了该方式的有效性。首次介绍并分析了应用在牙髓再生领域中不同预处理方式之间的侧重点,对应用于组织再生的多种预处理方式能否有效转化应用在牙髓再生的前景进行了展望,以期开辟出一条新的、完善的再生优化策略。
3.3 综述的局限性 ①目前外泌体的获取方式主要有超高速离心、试剂盒法、密度梯度离心、色谱法、免疫磁珠法以及过滤离心等,因超高速离心法获得的外泌体纯度较高且较其他方法拥有较少的小分子蛋白等杂质的残留,被普遍采用,牙髓再生领域中的相关文章也大多采用该方法,所以文章对其余外泌体的获取方式未进行讨论。②文章主要通过总结外泌体及其生物学特性,论述了外泌体对成血管、成神经和成牙分化的相关促进作用和机制以及最终的牙髓再生效果,但在再生过程还包括各类蛋白的生成、细胞间的相互转化等,因目前相关报道较少,文章并未作有关讨论。③目前应用于组织修复再生领域的预处理方式种类较多,但在牙髓再生领域中的预处理方式仅有炎症刺激、低氧、条件培养基以及三维培养,其余方式罕见报道,文章对上述预处理方式进行了详细的论述,介绍了各方式在牙髓再生中的具体效果,且对应用于组织再生的更多预处理方式能否转化应用在牙髓再生领域进行了相应展望,但未能充分且有力的对各种预处理方式的优缺点进行比较,对各预处理方式作用效果的相关机制也并未阐明,具体优缺点和机制还需要进行相关针对性后续研究进行探索和论证。
3.4 综述的重要意义 近年来,外泌体在牙髓再生领域已取得了一定的治疗效果,而经预处理衍生的外泌体也逐渐进入了研究视角。文章通过回顾大量文献发现经预处理衍生的外泌体其功能特性得到显著改善,在成牙分化、牙髓血管和神经再生等方面具有较好的促进作用,能有效提高牙髓再生效果。虽然研究者们通过应用不同预处理方式改善了牙髓再生的效果,但仍有一些问题亟待解决。比如除目前应用在牙髓再生领域中的炎症刺激、低氧诱导、条件培养基和三维培养方式以外,超声、机械刺激、辐射刺激和药物刺激等预处理条件下衍生的外泌体对组织修复再生具有较好的再生效果,在牙髓再生中其是否具有同样的促进效果,相关研究有待进一步深入探索与阐明;虽然预处理衍生的外泌体可以有效提高再生牙髓质量,但不同预处理方式衍生的外泌体参与牙髓再生的潜力不同,如何选择最为适宜的供体预处理方式以及具体的处理时间和浓度等条件从而达到更为理想的再生效果或将成为学者们下一步研究的方向。此综述通过总结外泌体促进牙髓再生的有关机制作用,结合应用在组织修复再生方面的供体细胞预处理方式,梳理外泌体及其不同预处理方式在牙髓再生研究领域现阶段中的进展,归纳预处理衍生的外泌体对牙髓再生的增强作用,展望了更多预处理方式能否应用在该领域,为后续外泌体更广阔的应用研究以及转化医学研究奠定基础。相信随着对外泌体和相关预处理方式的不断探索应用与研究,在不久的将来可以更好实现理想的牙髓再生。
3.5 课题组专家对未来的建议 外泌体在细胞核内体网中形成,以胞膜出芽的方式产生,其特性和来源细胞的培养微环境息息相关。通过探究来源细胞的生物学特性以及培养微环境寻找更优的外泌体预处理方式并阐明其作用机制,为今后外泌体的优化研究寻找新的“突破点”。在牙髓再生领域中,外泌体的预处理因素仍处于初期阶段,现有的预处理方式中如炎性刺激浓度、低氧的氧含量等部分问题和作用机制还需进一步完善,组织再生修复等领域的预处理方式能否转化应用在牙髓再生中,更多的未知方式和应用研究有待探索和完善。未来期望通过更加有针对性以及全面的研究提高外泌体的应用潜力,为将来牙髓牙本质的优化再生提供全新的研究策略和依据,为今后预处理的外泌体在组织修复再生等领域中的应用奠定基础,也为外泌体能够早日应用在临床提供方向。 中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
 #br#
#br#
文题释义:
经细胞预处理分泌的外泌体:细胞的培养环境高度影响着其分泌的外泌体生物学特性,通过改变细胞的培养环境以达到改善外泌体的功能特性的目的,从而优化外泌体在组织再生等领域中的治疗效果与应用。牙髓再生:通过调控干细胞的增殖、迁移和分化促进牙髓血管和神经的生成以及牙髓和牙本质样组织的形成,最终达到重建受损牙髓组织的结构和功能特性的目的。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
牙髓是位于髓腔内的疏松结缔组织,被坚硬的牙本质所包围。牙髓中的血管、淋巴管和神经仅通过根尖孔与根尖部的牙周组织相通连,一旦损伤很难自行恢复,导致牙髓病和根尖周病的发生。目前临床常规的治疗方法是根管治疗术,此技术虽然可以终止病变的发展保留患牙,却无法有效恢复牙髓的功能。失髓患牙失去营养往往容易发生牙折或者因无法感知外界刺激造成再次感染。因此,重建受损牙髓组织的结构和功能实现牙髓再生成为目前亟待解决的问题。可预见的以及可靠的干细胞定向分化是组织工程技术的核心。目前,干细胞在牙髓再生领域的应用受到了大量的关注,但同时干细胞治疗也存在一些如免疫排斥、干细胞遗传物质变异、体外扩增保存不易等问题。干细胞来源的外泌体因保留了干细胞的功能又避免了细胞移植相关的风险和限制,成为近年来牙髓再生领域研究的热点。
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||