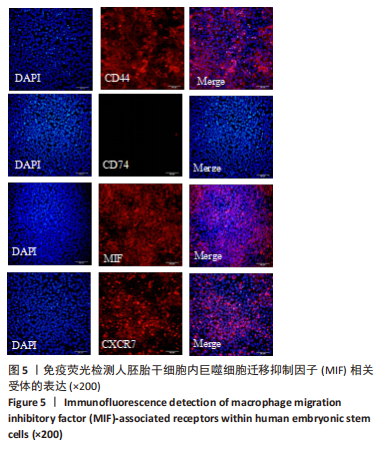
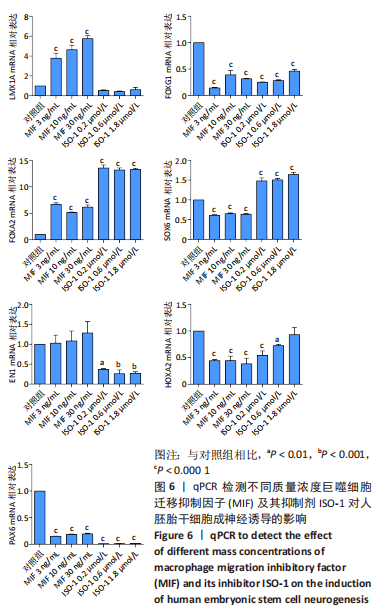
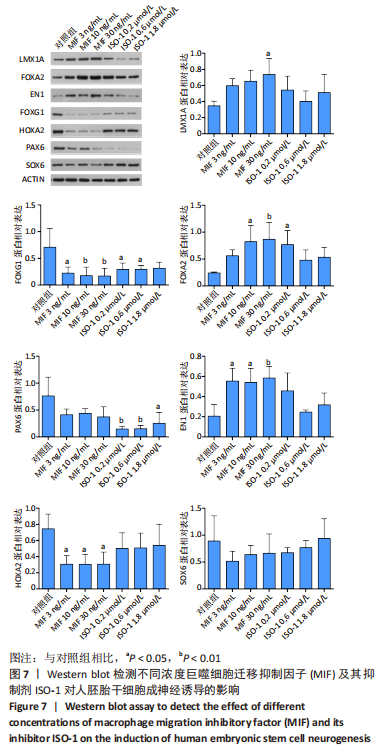
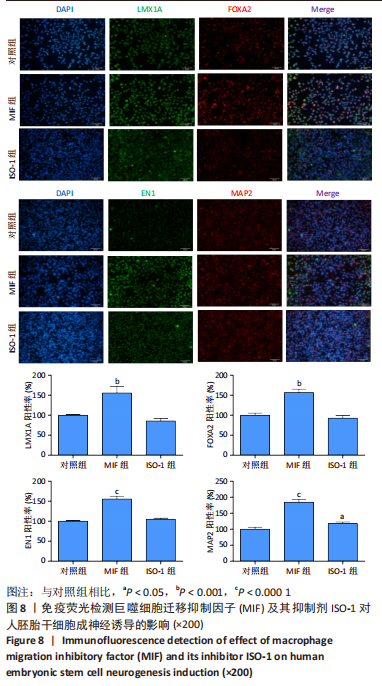
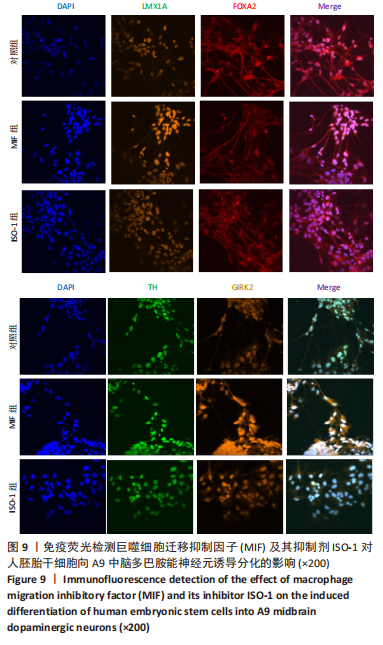
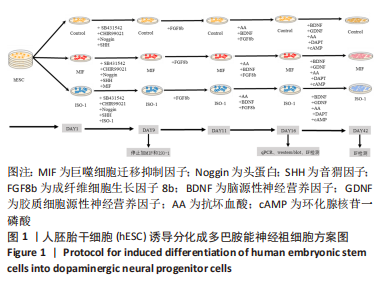
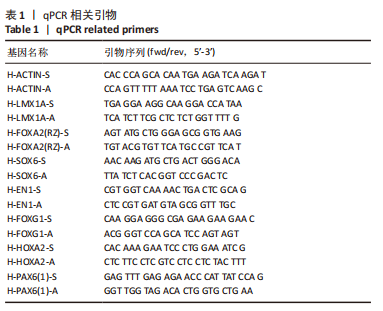
[1] TANG Y, HAN L, BAI X, et al. Intranasal Delivery of Bone Marrow Stromal Cells Preconditioned with Fasudil to Treat a Mouse Model of Parkinson’s Disease. Neuropsychiatr Dis Treat. 2020;16:249-262.
[2] FAN Y, WINANTO, NG SY. Replacing what’s lost: a new era of stem cell therapy for Parkinson’s disease. Transl Neurodegener. 2020;9:2.
[3] ARMSTRONG MJ, OKUN MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323(6):548-560.
[4] OLANOW CW, FACTOR SA, ESPAY AJ, et al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 2020;19(2):135-144.
[5] GAO L, NATH SC, JIAO X, et al. Post-Passage rock inhibition induces cytoskeletal aberrations and apoptosis in Human embryonic stem cells. Stem Cell Res. 2019;41:101641.
[6] 陈丽,胡兰,彭雅南,等.人多能干细胞向多巴胺能神经元分化:异质性的安全风险[J].中国组织工程研究,2019,23(1):118-124.
[7] 李光然,王伟.小分子化合物在干细胞神经分化中的研究进展[J].中国生物工程杂志,2018,38(3):76-80.
[8] KIRKEBY A, NOLBRANT S, TIKLOVA K, et al. Predictive Markers Guide Differentiation to Improve Graft Outcome in Clinical Translation of hESC-Based Therapy for Parkinson’s Disease. Cell Stem Cell. 2017; 20(1):135-148.
[9] SONG B, CHA Y, KO S, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest. 2020;130(2):904-920.
[10] NOLBRANT S, HEUER A, PARMAR M, et al. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat Protoc. 2017;12(9): 1962-1979.
[11] SAMATA B, DOI D, NISHIMURA K, et al. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat Commun. 2016;7:13097.
[12] ZHAO Z, MA Y, CHEN Z, et al. Effects of Feeder Cells on Dopaminergic Differentiation of Human Embryonic Stem Cells. Front Cell Neurosci. 2016;10:291.
[13] KEE N, VOLAKAKIS N, KIRKEBY A, et al. Single-Cell Analysis Reveals a Close Relationship between Differentiating Dopamine and Subthalamic Nucleus Neuronal Lineages. Cell Stem Cell. 2017;20(1):29-40.
[14] XIA N, FANG F, ZHANG P, et al. A Knockin Reporter Allows Purification and Characterization of mDA Neurons from Heterogeneous Populations. Cell Rep. 2017;18(10):2533-2546.
[15] BILSBORROW JB, DOHERTY E, TILSTAM PV, et al. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther Targets. 2019; 23(9):733-744.
[16] AL-ABED Y, VANPATTEN S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3(1):45-63.
[17] JANKAUSKAS SS, WONG DWL, BUCALA R, et al. Evolving complexity of MIF signaling. Cell Signal. 2019;57:76-88.
[18] OHTA S, MISAWA A, FUKAYA R, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival and proliferation of neural stem/progenitor cells. J Cell Sci. 2012;125(Pt 13):3210-3220.
[19] MILLER EJ, LI J, LENG L, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451(7178):578-582.
[20] SANTOS LL, MORAND EF. The role of macrophage migration inhibitory factor in the inflammatory immune response and rheumatoid arthritis. Wien Med Wochenschr. 2006;156(1-2):11-18.
[21] CONBOY L, VAREA E, CASTRO JE, et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol Psychiatry. 2011;16(5):533-547.
[22] LIU F, UCHUGONOVA A, KIMURA H, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10(5):830-839.
[23] OHTA S, MISAWA A, LEFEBVRE V, et al. Sox6 up-regulation by macrophage migration inhibitory factor promotes survival and maintenance of mouse neural stem/progenitor cells. PLoS One. 2013;8(9):e74315.
[24] PANMAN L, PAPATHANOU M, LAGUNA A, et al. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 2014;8(4):1018-1025.
[25] OLIVEIRA CS, DE BOCK CE, MOLLOY TJ, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Cancer. 2014;14:630.
[26] WEI QT, LIU BY, JI HY, et al. Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT axis in gliomas. Mol Ther Oncolytics. 2021;22:114-128.
[27] MOURTZI T, KAZANIS I. Endogenous versus exogenous cell replacement for Parkinson’s disease: where are we at and where are we going? Neural Regen Res. 2022;17(12):2637-2642.
[28] MESMAN S, VON OERTHEL L, SMIDT MP. Mesodiencephalic dopaminergic neuronal differentiation does not involve GLI2A-mediated SHH-signaling and is under the direct influence of canonical WNT signaling. PLoS One. 2014;9(5):e97926.
[29] NGUYEN HN, BYERS B, CORD B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8(3):267-280.
[30] FROM THE AMERICAN ASSOCIATION OF NEUROLOGICAL SURGEONS (AANS), AMERICAN SOCIETY OF NEURORADIOLOGY (ASNR), CARDIOVASCULAR AND INTERVENTIONAL RADIOLOGY SOCIETY OF EUROPE (CIRSE), et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018;13(6):612-632.
[31] POULIN JF, GAERTNER Z, MORENO-RAMOS OA, et al. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 2020;43(3):155-169.
[32] LA MANNO G, GYLLBORG D, CODELUPPI S, et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell. 2016;167(2):566-580.e19.
[33] MA T, WANG C, WANG L, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16(11):1588-1597.
[34] MANUEL MN, MI D, MASON JO, et al. Regulation of cerebral cortical neurogenesis by the Pax6 transcription factor. Front Cell Neurosci. 2015;9:70.
[35] COOPER O, HARGUS G, DELEIDI M, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45(3):258-266.
[36] HÉBERT JM, FISHELL G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9(9):678-685.
[37] HETTIGE NC, ERNST C. FOXG1 Dose in Brain Development. Front Pediatr. 2019;7:482.
[38] TÜMPEL S, MACONOCHIE M, WIEDEMANN LM, et al. Conservation and diversity in the cis-regulatory networks that integrate information controlling expression of Hoxa2 in hindbrain and cranial neural crest cells in vertebrates. Dev Biol. 2002;246(1):45-56.
[39] DAVENNE M, MACONOCHIE MK, NEUN R, et al. Hoxa2 and Hoxb2 control dorsoventral patterns of neuronal development in the rostral hindbrain. Neuron. 1999;22(4):677-691.
[40] XIE J, YANG L, TIAN L, et al. Macrophage Migration Inhibitor Factor Upregulates MCP-1 Expression in an Autocrine Manner in Hepatocytes during Acute Mouse Liver Injury. Sci Rep. 2016;6:27665.
[41] NISHINO T, BERNHAGEN J, SHIIKI H, et al. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Mol Med. 1995;1(7):781-788.
[42] EICKHOFF R, WILHELM B, RENNEBERG H, et al. Purification and characterization of macrophage migration inhibitory factor as a secretory protein from rat epididymis: evidences for alternative release and transfer to spermatozoa. Mol Med. 2001;7(1):27-35.
[43] PYLE ME, KORBONITS M, GUEORGUIEV M, et al. Macrophage migration inhibitory factor expression is increased in pituitary adenoma cell nuclei. J Endocrinol. 2003;176(1):103-110.
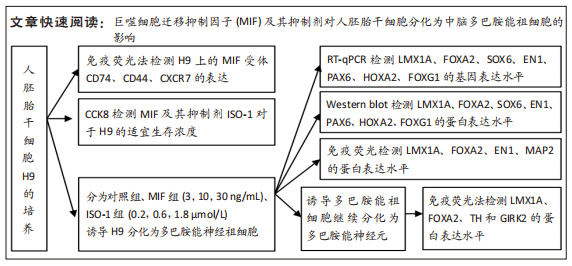
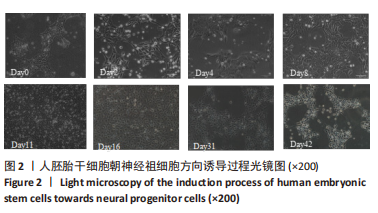
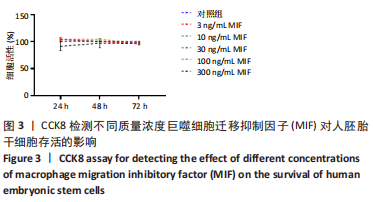
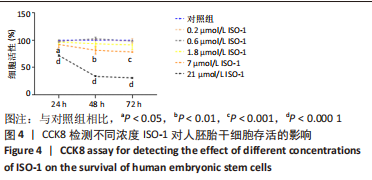
[44] 魏艳召,郑晓晗,高仕君,等.人胚胎干细胞自分泌巨噬细胞迁移抑制因子及受体表达[J].中国组织工程研究,2023,27(1):34-41.
[45] 郑晓晗,魏艳召,黄婷,等.巨噬细胞迁移抑制因子对干细胞的作用[J].中国组织工程研究,2023,27(15):2395-2403.
[46] CHATTERJEE M, BORST O, WALKER B, et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ Res. 2014;115(11):939-949.
[47] AMIN MA, HAAS CS, ZHU K, et al. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood. 2006;107(6): 2252-2261.
[48] BERNHAGEN J, KROHN R, LUE H, et al.MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment.Nat Med. 2007;13(5):587-596.
[49] SCHWARTZ V, LUE H, KRAEMER S, et al.A functional heteromeric MIF receptor formed by CD74 and CXCR4.FEBS Lett. 2009;583(17):2749-2757.
[50] ALAMPOUR-RAJABI S, EL BOUNKARI O, ROT A, et al. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. 2015;29(11): 4497-4511.
[51] LEVOYE A, BALABANIAN K, BALEUX F, et al.CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085-6093.
[52] RAJAGOPAL S, KIM J, AHN S, et al.Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107(2):628-632. |