[1] LI L, LU H, ZHAO Y, et al. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: A review and new perspectives. Mater Sci Eng C Mater Biol Appl. 2019;98:1241-1251.
[2] KELLY CN, LIN AS, LEGUINECHE KE, et al. Functional repair of critically sized femoral defects treated with bioinspired titanium gyroid-sheet scaffolds. J Mech Behav Biomed Mater. 2021;116:104380.
[3] DEKKER TJ, STEELE JR, FEDERER AE, et al. Use of Patient-Specific 3D-Printed Titanium Implants for Complex Foot and Ankle Limb Salvage, Deformity Correction, and Arthrodesis Procedures. Foot Ankle Int. 2018;39(8):916-921.
[4] LIU T, FANG W, WU G, et al. Low Dose BMP2-Doped Calcium Phosphate Graft Promotes Bone Defect Healing in a Large Animal Model. Front Cell Dev Biol. 2020; 8:613891.
[5] MARINO JT, ZIRAN BH. Use of solid and cancellous autologous bone graft for fractures and nonunions. The Orthopedic clinics of North America. 2010;41(1): 15-26.
[6] ZHAI P, PENG X, LI B, et al. The application of hyaluronic acid in bone regeneration. Int J Biol Macromol. 2020;151:1224-1239.
[7] AMINI Z, LARI R. A systematic review of decellularized allograft and xenograft-derived scaffolds in bone tissue regeneration. Tissue Cell. 2021;69:101494.
[8] COHEN DJ, SCOTT KM, KULKARNI AN, et al. Acellular mineralized allogenic block bone graft does not remodel during the 10 weeks following concurrent implant placement in a rabbit femoral model. Clin Oral Implants Res. 2020;31(1):37-48.
[9] CAMPANA V, MILANO G, PAGANO E, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014; 25(10):2445-2461.
[10] RODDY E, DEBAUN MR, DAOUD-GRAY A, et al. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28(3):351-362.
[11] ZHOU H, YANG L, GBURECK U, et al. Monetite, an important calcium phosphate compound-Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021;127:41-55.
[12] YUAN B, WANG Z, ZHAO Y, et al. In Vitro and In Vivo Study of a Novel Nanoscale Demineralized Bone Matrix Coated PCL/β-TCP Scaffold for Bone Regeneration. Macromol Biosci. 2021;21(3):e2000336.
[13] CHARBONNIER B, MANASSERO M, BOURGUIGNON M, et al. Custom-made macroporous bioceramic implants based on triply-periodic minimal surfaces for bone defects in load-bearing sites. Acta Biomater. 2020;109:254-266.
[14] STASTNY P, SEDLACEK R, SUCHY T, et al. Structure degradation and strength changes of sintered calcium phosphate bone scaffolds with different phase structures during simulated biodegradation in vitro. Mater Sci Eng C Mater Biol Appl. 2019;100:544-553.
[15] AL-KETAN O, LEE DW, ROWSHAN R, et al. Functionally graded and multi-morphology sheet TPMS lattices: Design, manufacturing, and mechanical properties. J Mech Behav Biomed Mater. 2020;102:103520.
[16] KELLY CN, FRANCOVICH J, JULMI S, et al. Fatigue behavior of As-built selective laser melted titanium scaffolds with sheet-based gyroid microarchitecture for bone tissue engineering. Acta Biomater. 2019;94:610-626.
[17] RAJAGOPALAN S, ROBB RA. Schwarz meets Schwann: design and fabrication of biomorphic and durataxic tissue engineering scaffolds. Med Image Anal. 2006; 10(5):693-712.
[18] HE L, LIU X, RUDD C. Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering. Polymers (Basel). 2021;13(2):270.
[19] PARÉ A, CHARBONNIER B, TOURNIER P, et al. Tailored Three-Dimensionally Printed Triply Periodic Calcium Phosphate Implants: A Preclinical Study for Craniofacial Bone Repair. ACS Biomater Sci Eng. 2020;6(1):553-563.
[20] 贾涛.钛基层状多孔材料的制备及压缩力学性能研究[D].西安:西安理工大学,2020.
[21] CHENG Q, HUANG C, TOMSIA AP. Freeze Casting for Assembling Bioinspired Structural Materials. Adv Mater. 2017;29(45). doi: 10.1002/adma.201703155.
[22] KE Z, YANG L, WU H, et al. Evaluation of in vitro and in vivo antitumor effects of gambogic acid-loaded layer-by-layer self-assembled micelles. Int J Pharm. 2018; 545(1-2): 306-317.
[23] YANG Y, LI X, CHU M, et al. Electrically assisted 3D printing of nacre-inspired structures with self-sensing capability. Sci Adv. 2019;5(4):eaau9490.
[24] BARBER H, KELLY CN, NELSON K, et al. Compressive anisotropy of sheet and strut based porous Ti-6Al-4V scaffolds. J Mech Behav Biomed Mater. 2021;115:104243.
[25] ALI D. Effect of scaffold architecture on cell seeding efficiency: A discrete phase model CFD analysis. Comput Biol Med. 2019;109:62-69.
[26] ZANKOVIC S, SEIDENSTUECKER M, PRALL WC, et al. A Method for the Evaluation of Early Osseointegration of Implant Materials Ex Vivo: Human Bone Organ Model. Materials (Basel). 2021;14(11):3001.
[27] LIU Y, YANG S, CAO L, et al. Facilitated vascularization and enhanced bone regeneration by manipulation hierarchical pore structure of scaffolds. Mater Sci Eng C Mater Biol Appl. 2020;110:110622.
[28] FREEMAN FE, BROWE DC, NULTY J, et al. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur Cells Mater. 2019; 38:168-187.
[29] OLADAPO BI, ZAHEDI SA, ISMAIL SO, et al. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material- A review. Colloids Surf B Biointerfaces. 2021;203:111726.
[30] DAVOODI E, MONTAZERIAN H, ESMAEILIZADEH R, et al. Additively Manufactured Gradient Porous Ti-6Al-4V Hip Replacement Implants Embedded with Cell-Laden Gelatin Methacryloyl Hydrogels. ACS Appl Mater Interfaces. 2021;13(19):22110-22123.
[31] MANZOOR F, GOLBANG A, JINDAL S, et al. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J Mech Behav Biomed Mater. 2021;121:104601.
[32] 耿鹏.聚醚醚酮及其复合材料增材制造机理与实验研究[D].长春:吉林大学, 2019.
[33] CHANG HY, TUAN WH, LAI PL. Biphasic ceramic bone graft with biphasic degradation rates. Mater Sci Eng C Mater Biol Appl. 2021;118:111421.
[34] WANG C, LAI J, LI K, et al. Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact Mater. 2021; 6(1):137-145.
[35] ZHONG L, CHEN J, MA Z, et al. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale. 2020;12(48):24437-24449.
[36] MELCHELS F PW, BARRADAS AMC, VAN BLITTERSWIJK CA, et al. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010;6(11):4208-4217.
[37] HU J, WANG S, LI B, et al. Efficient Representation and Optimization for TPMS-Based Porous Structures. IEEE Trans Vis Comput Graph. 2020. doi: 10.1109/TVCG. 2020.3037697.
[38] ZADPOOR AA. Mechanical performance of additively manufactured meta-biomaterials. Acta Biomater. 2019;85:41-59.
[39] SANTOS J, PIRES T, GOUVEIA BP, et al. On the permeability of TPMS scaffolds. J Mech Behav Biomed Mater. 2020;110:103932.
[40] MA S, SONG K, LAN J, et al. Biological and mechanical property analysis for designed heterogeneous porous scaffolds based on the refined TPMS. J Mech Behav Biomed Mater. 2020;107:103727.
[41] HENKEL J, WOODRUFF MA, EPARI DR, et al. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res. 2013;1(3):216-248.
[42] 周丽超.基于三周期极小曲面的模型空间剖分研究[D].大连:大连理工大学, 2019.
[43] HA M, ATHIRASALA A, TAHAYERI A, et al. Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro. Dent Mater. 2020;36(1):88-96.
[44] HAYASHI K, MUNAR ML, ISHIKAWA K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration. Mater Sci Eng C Mater Biol Appl. 2020;111:110848.
[45] GUPTE MJ, SWANSON WB, HU J, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1-11.
[46] DIAO J, OUYANG J, DENG T, et al. 3D-Plotted Beta-Tricalcium Phosphate Scaffolds with Smaller Pore Sizes Improve In Vivo Bone Regeneration and Biomechanical Properties in a Critical-Sized Calvarial Defect Rat Model. Adv Healthc Mater. 2018; 7(17):e1800441.
[47] LI L, SHI J, ZHANG K, et al. Early osteointegration evaluation of porous Ti6Al4V scaffolds designed based on triply periodic minimal surface models. J Orthop Translat. 2019;19:94-105.
[48] 刁静静.磷酸钙生物活性陶瓷支架孔尺寸的精确调控及骨再生修复效果[D].广州:华南理工大学,2019.
|

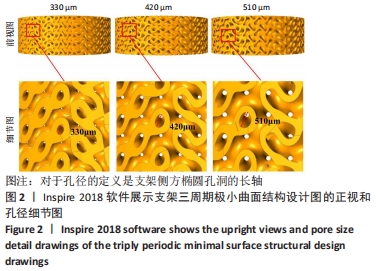
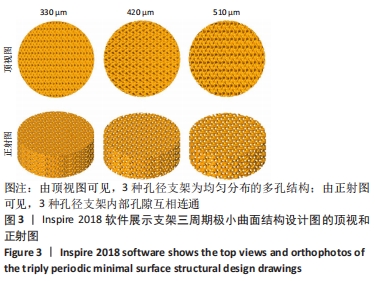

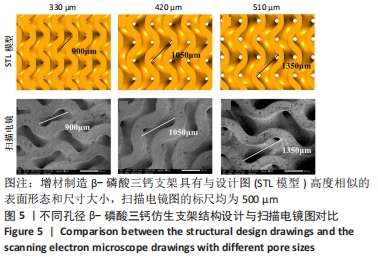
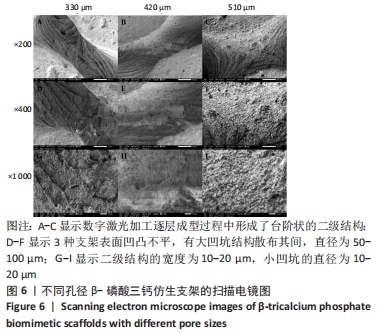
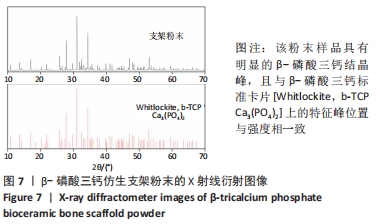
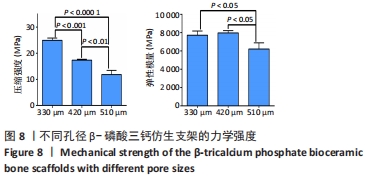
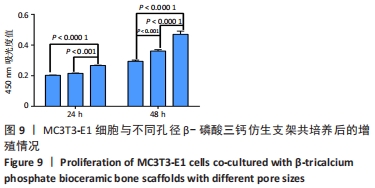
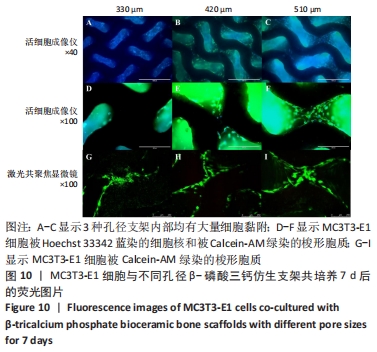



 MELCHELS等[36]的研究证实,与具有相似孔隙率和孔径的随机孔结构相比,三周期极小曲面结构植入物的渗透性更高,其中三周期极小曲面G曲面多孔结构具有最好的间充质干细胞浸润效果。实验选择三周期极小曲面G表面作为骨支架结构,曲率C为三周期极小曲面结构壁厚的调控参数。x,y,z的范围是[-2π,2π],其中2π是系数,代表三周期极小曲面结构的周期为1,周期为三周期极小曲面结构孔径的调控参数。通过调节支架的壁厚和孔径可达到对松质骨密度、弹性模量和渗透率等方面的精确仿生。使用王胜法教授课题组内部自主开发的多孔结构优化方法[37],将设计的支架厚度(实验中是200 μm)赋予三周期极小曲面方程,生成三维的三周期极小曲面骨支架结构设计。
MELCHELS等[36]的研究证实,与具有相似孔隙率和孔径的随机孔结构相比,三周期极小曲面结构植入物的渗透性更高,其中三周期极小曲面G曲面多孔结构具有最好的间充质干细胞浸润效果。实验选择三周期极小曲面G表面作为骨支架结构,曲率C为三周期极小曲面结构壁厚的调控参数。x,y,z的范围是[-2π,2π],其中2π是系数,代表三周期极小曲面结构的周期为1,周期为三周期极小曲面结构孔径的调控参数。通过调节支架的壁厚和孔径可达到对松质骨密度、弹性模量和渗透率等方面的精确仿生。使用王胜法教授课题组内部自主开发的多孔结构优化方法[37],将设计的支架厚度(实验中是200 μm)赋予三周期极小曲面方程,生成三维的三周期极小曲面骨支架结构设计。