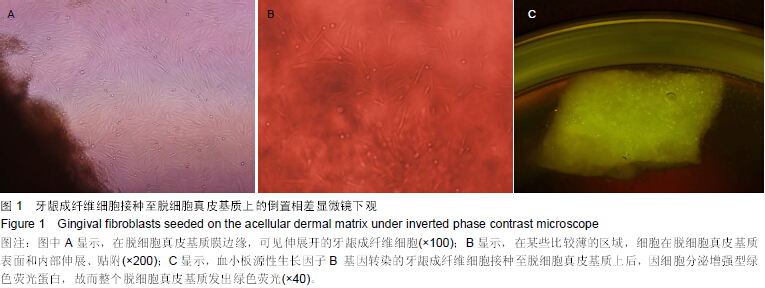
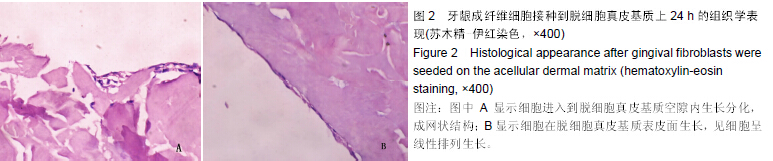
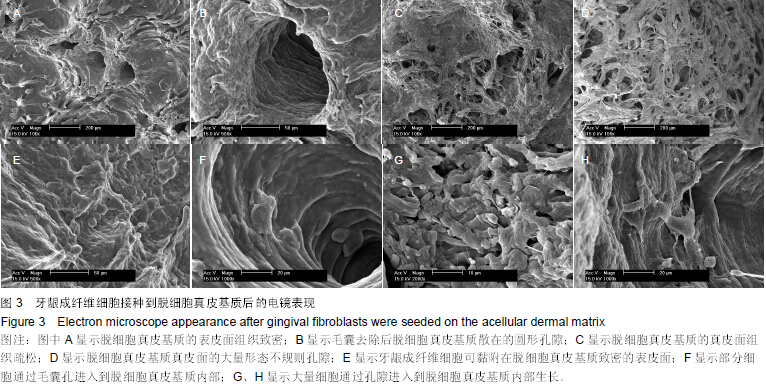
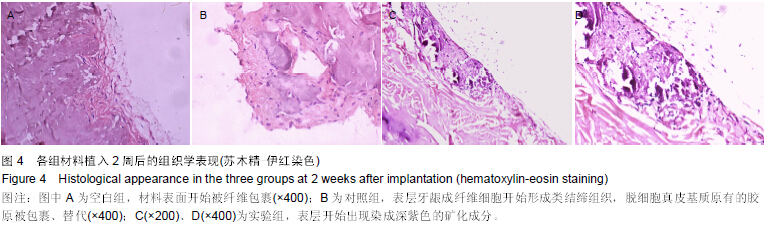
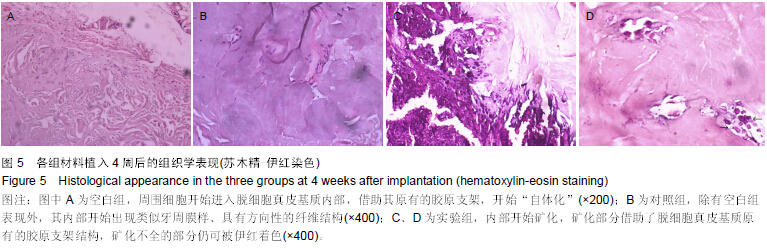
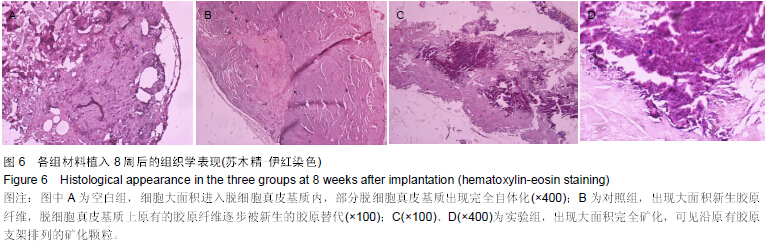
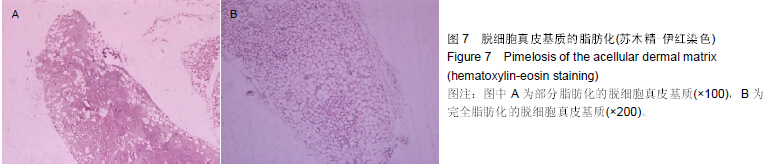
| [1] Heldin CH,Westermark B. Mechanism of action and in vivo role of platelet -derived growth factor. Physiol Rev.1999;79(4): 1283-1316.
[2] 钟泉,闫福华,李艳芬,等.hPDGF-B基因转染对Beagle犬牙龈成纤维细胞生物学活性的影响[J].福建医科大学学报,2013,47(6): 329-334.
[3] 钟泉,闫福华,李艳芬,等.人血小板源性生长因子-B基因转染犬牙龈成纤维细胞的瞬时表达检测[J].华西口腔医学杂志,2011, 29(2):214-219.
[4] 高扬,王景云.脱细胞真皮基质在口腔医学领域中的应用进展[J].口腔医学研究,2006,22(2):214-216.
[5] Taylor JB,Gerlach RC,Herold RW,et al.A modified tensionless gingival grafting technique using acellular dermal matrix.Int J Periodontics Restorative Dent. 2010;30(5):513-521.
[6] 钟泉,闫福华,李艳芬,等.Beagle犬牙龈成纤维细胞的体外分离培养[J].中国组织工程研究与临床康复,2008,12(50):9801-9805
[7] Michaeli-Geller G,Zigdon-Giladi H. Bone regeneration induced by stem cells--recent research and future outlook. Refuat Hapeh Vehashinayim.2015;32(1):13-20.
[8] 殷春一,章锦才.组织工程[J].广东牙病防治,2002,10(2): 151-153.
[9] Al-Hezaimi K, Rudek I,AI-Hamdan KS,et al.Efficacy of acellular dermal matrix and coronally advanced flaps for the treatment of induced gingival recession defects: a histomorphometric study in dogs.J Periodontol.2013; 84(8): 1172-1179.
[10] Cummings LC, Kaldahl WB, Allen EP.Histologic Evaluation of autogenous connective tissue and acellular dermal matrix grafts in humans.J Periodontol.2005;76(2):178-186.
[11] 刘德伍,李国辉,邹萍,等.表皮细胞、成纤维细胞复合脱细胞真皮基质构建组织工程皮肤[J].中国临床康复,2004,8(8): 1439-1441.
[12] 赖红昌,戴烨扬,张志勇.应用牙龈角质细胞构建组织工程化牙龈的实验研究[J]. 中国口腔颌面外科杂志,2007,5(1):46-49.
[13] Novaes AB Jr, Marchesan JT,Macedo GO,et al.Effect of in vitro Gingical Fibroblast Seeding on the in vivo Incorporation of Acellular Dermal Matrix Allografts in Dogs. J Periodontol. 2007;78(2):296-303.
[14] 宁方刚,张国安,金培勇.利用免疫学方法检测脱细胞真皮基质的细胞碎片残留[J].中华烧伤杂志,2007,23(1):73.
[15] Riccio V,Raglone FD,Marrone G,et al.Cultures of human embryonic osteoblasts. Clin Orthop.1994;308(1):73-75.
[16] Matsumura K,Hyon SH,Nakajima N,et al.Effects on gingival cells of hydroxyapatite immobilized on poly(ethylene-co-vinyl alcohol).J Biomed Mater Res A.2007;82(2):288-295.
[17] Nakajima K,Abe T,Tanaka M,et al. Periodontal tissue engineering by transplantation of multilayered sheets of phenotypically modified gingival fibroblasts.J Periodontal Res. 2008;43(6):681-688.
[18] 张韶君,彭凤梅,章锦才.rhBMP-2对人牙龈成纤维细胞体外矿化能力的影响[J].广东牙病防治,2004,12(1):15-17.
[19] Jin Q,Anusaksathien O,Webb SA,et al.Gene therapy of bone morphogenetic protein for periodontal tissue engineering.J Periodontol.2003;74(2):202-213.
[20] 赵川江,章锦才,徐琛蓉,等.人釉原蛋白基因转染对人牙龈成纤维细胞生物学活性的影响[J].中山大学学报:医学版,2006,27(B04): 25-27.
[21] Krebsbach PH,Renny KG,Franceshi RT,et al.Gene therapy-directed osteogenesis: BMP-7 transduced human fibroblasts from bone in vivo.Hum Gene.2000;11(8): 1201-1210.
[22] Rutherford RB, Racenis P, Fatherazi S, et al. Bone formation by BMP -7-transduced human gingival keratinocytes.J Dent Res.2003;82(4):293-297.
[23] Hakki SS,Wang D,Franceschi RT,et al.Bone sialoprotein gene transfer to periodontal ligament cells may not be sufficient to promote mineralization in vitro or in vivo. J Periodontol.2006; 77(2):167-173.
[24] Lin Z,Sugai JV,Jin Q,et al.Platelet-derived growth factor –B gene delivery sustains gingival fibroblast signal transduction.J Periodont Res.2008;43(4):440-449.
[25] Anusaksathien O,Webb SA,Jin QM,et al.Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng.2003;9(4):745-755.
[26] Qiu QQ,Connor J.Effects of γ-irradiation, storage and hydration on osteoinductivit of DBM and DBM/AM composite. J Biomed Mater Res A.2008;87(2):373-379. |