中国组织工程研究 ›› 2013, Vol. 17 ›› Issue (14): 2578-2585.doi: 10.3969/j.issn.2095-4344.2013.14.016
• 干细胞培养与分化 stem cell culture and differentiation • 上一篇 下一篇
人脐血和外周血内皮祖细胞的分离培养和鉴定
李欢欢1,赵丽静1,何 旭2,王 娟1,刘亢丁1
- 1吉林大学第一医院神经内科, 吉林省长春市 130021
2吉林大学基础医学院病理生物学教育部重点实验室,吉林省长春市 130021
-
收稿日期:2012-03-17修回日期:2012-05-22出版日期:2013-04-02发布日期:2013-04-02 -
通讯作者:刘亢丁,博士,教授,吉林大学第一医院神经内科,吉林省长春市 130021 -
作者简介:李欢欢★,女,1984年生,河南省灵宝市人,汉族,2010年吉林大学毕业,硕士,医师,现工作于解放军第四军医大学唐都医院神经内科,主要从事脑血管病研究。
Endothelial progenitor cells from human umbilical cord blood and peripheral blood: Isolation, culture and identification
Li Huan-huan1, Zhao Li-jing1, He Xu2, Wang Juan1, Liu Kang-ding1
- 1 Department of Neurology, the First Hospital of Jilin University, Changchun 130021, Jilin Province, China
2 Department of Pathology, Key Laboratory of the Ministry of Education, School of Basic Medical Science, Jilin University, Changchun 130021, Jilin Province, China
-
Received:2012-03-17Revised:2012-05-22Online:2013-04-02Published:2013-04-02 -
Contact:Liu Kang-ding, Doctor, Professor, Department of Neurology, the First Hospital of Jilin University, Changchun 130021, Jilin Province, China kangdingliu@163.com -
About author:Li Huan-huan★, Master, Physician, Department of Neurology, the First Hospital of Jilin University, Changchun 130021, Jilin Province, China huanhuan19840620@163.com
摘要:
背景:国内外有关内皮祖细胞的分离培养和鉴定方面的文章很多,但是人脐血和外周血内皮祖细胞的鉴别方面文章不多。 目的:从人脐血和外周血中分离出内皮祖细胞,并对其进行培养和鉴定。 方法:选取密度梯度离心法从脐血和外周血中分离获得单个核细胞,按照1× 106/cm2的浓度种植于预先铺有纤维连接蛋白的培养板中,用含血管内皮生长因子的M199培养基进行诱导培养。 结果与结论:人脐血和外周血中存在内皮祖细胞,浓度梯度离心联合贴壁筛选获得的单个核细胞在血管内皮生长因子的诱导培养下可分化成内皮祖细胞,内皮祖细胞表达CD34、CD133、CD105、KDR和CD31,能吞噬乙酰化低密度脂蛋白,结合荆豆凝集素1,它们可作为体外分选内皮祖细胞的标志。
中图分类号:
引用本文
李欢欢,赵丽静,何 旭,王 娟,刘亢丁. 人脐血和外周血内皮祖细胞的分离培养和鉴定[J]. 中国组织工程研究, 2013, 17(14): 2578-2585.
Li Huan-huan, Zhao Li-jing, He Xu, Wang Juan, Liu Kang-ding. Endothelial progenitor cells from human umbilical cord blood and peripheral blood: Isolation, culture and identification[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(14): 2578-2585.
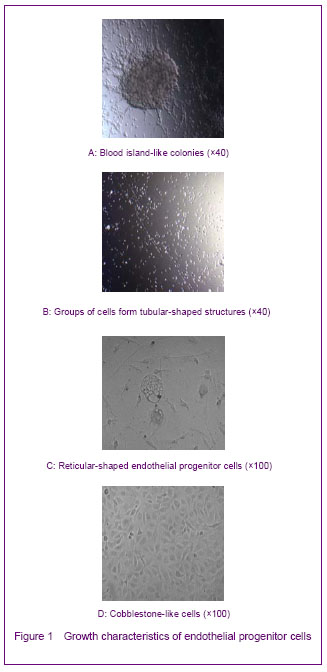
The growth characteristics of endothelial progenitor cells cultured in vitro included: time delay; blood island-like cell colony that composed of round cells and flat cells (Figure 1A); cable-like and mesh shapes connected end-to-end (Figures 1B, C); and cobblestone-like shapes (Figure 1D). These characteristics resembled those seen during the formation of the original vascular network in embryonic development, indicating that endothelial progenitor cells may participate in postnatal angiogenesis.
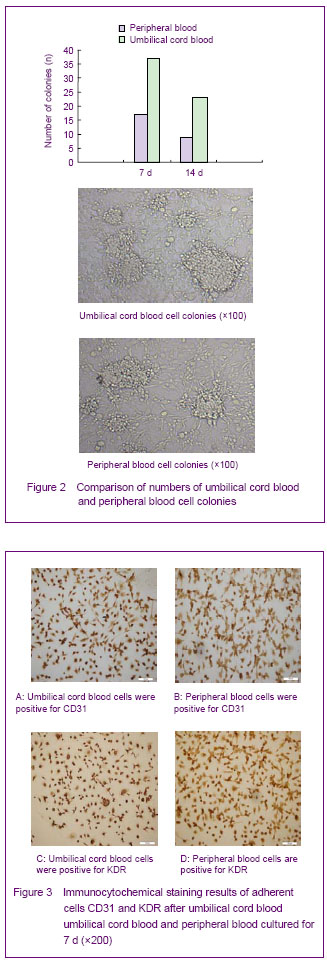
The numbers of umbilical cord blood and peripheral blood cell colonies were calculated at 7 and 14 days. Under the microscope, the cell colonies disappeared gradually with the increasing of culture time. There were more umbilical cord blood cell colonies than peripheral blood cell colonies on 7 and 14 days (Figure 2).
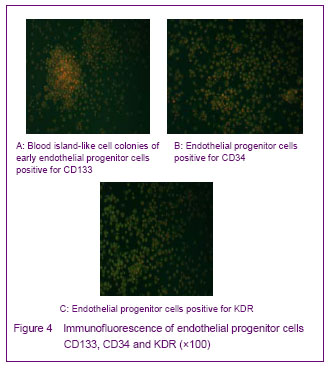
Adherent cells stained positively for CD31 and KDR on day 7. Staining in the endothelial progenitor cells was localized in the cell membrane (Figure 3).
Confocal laser scanning microscopy indicated that the cells that took up carbocyanine perchlorate labeled low density lipoprotein and bound Ulex europaeus agglutinin 1 were differentiating endothelial progenitor cells. Over 95% of endothelial progenitor cells showed double- positive staining with these reagents on day 10 (Figure 5).
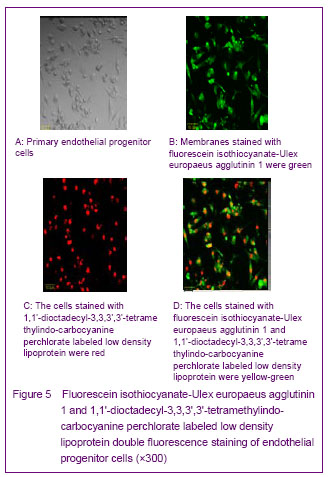
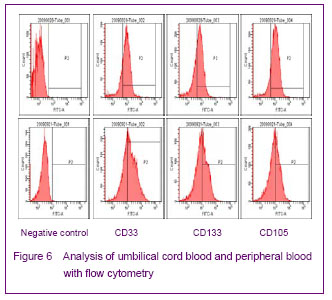
Flow cytometry analysis of endothelial progenitor cells showed that expression rates of CD34, CD133 and CD105 were 73.2%, 65.0% and 82.1%, respectively, for umbilical cord blood, and 60.1%, 47.6%, and 45.1%, respectively, for peripheral blood (Figure 6).
| [1] Yip HK, Chang LT, Chang WN, et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39(1):69-74.[2] He TR, Leslie AS, Harrington S, et al. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries transplantation. Stroke. 2004;35(10):2378-2384.[3] Patrick AU, Laurence MD, Dan GD, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111(3):1302-1305.[4] Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032-3039.[5] Urao N, Inomata H, Razvi M, et al. Role of nox22based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212-220.[6] Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science.1997;275:964-967.[7] Risau W, Flamme I. Vasculogenesis. Ann Rev Cell Dev Biol. 1995;11:73-91.[8] Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68(4):484-492.[9] Fei XH, Ye HH, Ruan LM, et al. Gender differences in adult circulating endothelial progenitor cells (EPCs) and effect of estradiol on arousing of EPCs in vitro. Zhonghua Bingli Shengli Zazhi. 2012;28(3):393-397.[10] Ge XB, Hu HJ, Deng FS, et al. SDF-1 combined with peripheral blood endothelial progenitor cells transplantation for the treatment of hindlimb ischemia in nude mice. Zhonghua Putong Waike Zazhi. 2011;26(7):584-588.[11] Ahrens I, Domeij H, Topcic D, et al. Successful in vitro expansion and differentiation of cord blood derived CD34+ cells into early endothelial progenitor cells reveals highly differential gene expression. PloS ONE. 2011;6(8): 23210.[12] Wyler von Ballmoos M, Yang Z, Völzmann J, et al. Endothelial progenitor cells induce a phenotype shift in differentiated endothelial cells towards PDGF/ PDGFβ axis-mediated angiogenesis. PloS ONE. 2010;5(11):e14107.[13] Zhang LJ, Liu WX, Chen YD, et al. Proliferation, migration and apoptosis activities of endothelial progenitor cells in acute coronary syndrome. Chin Med J (Engl). 2010; 123(19):2655-2661.[14] Rehman J, Li J, Orschell CM, et al. Peripheral blood endothelial progenitor cells\are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164-1169.[15] Gehling UM, Ergun S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC1332positive p rogenitor cells. Blood. 2000;95:3106-3112.[16] Hirschi KK,Ingram DA ,Yoder MC. Arterioscler Thromb Vasc Biol. 2008,28:1584.[17] Hristov M, Weber C. Endothelial progenitor cells: characterization,pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498-508.[18] Choi K, Kennedy M, Kazarov A, et al. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725-732.[19] Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002-5012.[20] Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-958.[21] Hur J, Yoon CH, Kim HS, et al. Characteristic of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288-293.[22] Yao G, Liu ZH, Zheng C, et al. Evaluation of renal vascular lesions using circulating endothelial cell in patients with lupus nephritis. Rheumatology(Oxford). 2008;47:432-436. [23] Bagley RG, Rouleau C, St Martin T, et al. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther. 2008;7: 2536-2546. |
| [1] | 蒲 锐, 陈子扬, 袁凌燕. 不同细胞来源外泌体保护心脏的特点与效应[J]. 中国组织工程研究, 2021, 25(在线): 1-. |
| [2] | 张秀梅, 翟运开, 赵 杰, 赵 萌. 类器官模型国内外数据库近10年文献研究热点分析[J]. 中国组织工程研究, 2021, 25(8): 1249-1255. |
| [3] | 王正东, 黄 娜, 陈婧娴, 郑作兵, 胡鑫宇, 李 梅, 苏 晓, 苏学森, 颜 南. 丁酸钠抑制氟中毒可诱导小胶质细胞活化及炎症因子表达增多[J]. 中国组织工程研究, 2021, 25(7): 1075-1080. |
| [4] | 汪显耀, 关亚琳, 刘忠山. 提高间充质干细胞治疗难愈性创面的策略[J]. 中国组织工程研究, 2021, 25(7): 1081-1087. |
| [5] | 万 然, 史 旭, 刘京松, 王岩松. 间充质干细胞分泌组治疗脊髓损伤的研究进展[J]. 中国组织工程研究, 2021, 25(7): 1088-1095. |
| [6] | 廖成成, 安家兴, 谭张雪, 王 倩, 刘建国. 口腔鳞状细胞癌干细胞的治疗靶点及应用前景[J]. 中国组织工程研究, 2021, 25(7): 1096-1103. |
| [7] | 谢文佳, 夏天娇, 周卿云, 刘羽佳, 顾小萍. 小胶质细胞介导神经元损伤在神经退行性疾病中的作用[J]. 中国组织工程研究, 2021, 25(7): 1109-1115. |
| [8] | 李珊珊, 郭笑霄, 尤 冉, 杨秀芬, 赵 露, 陈 曦, 王艳玲. 感光细胞替代治疗视网膜变性疾病[J]. 中国组织工程研究, 2021, 25(7): 1116-1121. |
| [9] | 焦 慧, 张一宁, 宋雨晴, 林 宇, 王秀丽. 乳腺癌类器官研究进展及临床应用前景[J]. 中国组织工程研究, 2021, 25(7): 1122-1128. |
| [10] | 王诗琦, 张金生. 中医药调控缺血缺氧微环境对骨髓间充质干细胞增殖、分化及衰老的影响[J]. 中国组织工程研究, 2021, 25(7): 1129-1134. |
| [11] | 曾燕华, 郝延磊. 许旺细胞体外培养及纯化的系统性综述[J]. 中国组织工程研究, 2021, 25(7): 1135-1141. |
| [12] | 孔德胜, 何晶晶, 冯宝峰, 郭瑞云, Asiamah Ernest Amponsah, 吕 飞, 张舒涵, 张晓琳, 马 隽, 崔慧先. 间充质干细胞修复大动物模型脊髓损伤疗效评价的Meta分析[J]. 中国组织工程研究, 2021, 25(7): 1142-1148. |
| [13] | 侯婧瑛, 于萌蕾, 郭天柱, 龙会宝, 吴 浩. 缺氧预处理激活HIF-1α/MALAT1/VEGFA通路促进骨髓间充质干细胞生存和血管再生[J]. 中国组织工程研究, 2021, 25(7): 985-990. |
| [14] | 史洋洋, 秦英飞, 吴福玲, 何 潇, 张雪静. 胎盘间充质干细胞预处理预防小鼠毛细支气管炎[J]. 中国组织工程研究, 2021, 25(7): 991-995. |
| [15] | 梁学奇, 郭黎姣, 陈贺捷, 武 杰, 孙雅琪, 邢稚坤, 邹海亮, 陈雪玲, 吴向未. 泡状棘球绦虫原头蚴抑制骨髓间充质干细胞向成纤维细胞的分化[J]. 中国组织工程研究, 2021, 25(7): 996-1001. |
Endothelial progenitor cells are multipotential cells present in the fetal liver, umbilical cord blood, and adult peripheral blood circulation that have the ability to differentiate into endothelial cells. Endothelial progenitor cells can predict the severity of vascular injury in the early stage[1], and they participate in the repair of endothelial damage[2], formation of new blood vessels[3], promotion of nerve regeneration[4], and prevention and treatment of thrombosis and lumen restenosis following stent implantation. Endothelial progenitor cells also represent a good prospect for the construction of tissue-engineered microvascular networks[5]. Endothelial progenitor cells have been increasingly used in the treatment of vascular diseases, and this is one of the most promising therapies for vascular diseases.
Cytology experiment in vitro.
The experiment was completed in the Pathological Laboratory, Norman Bethune University of Medical Science from March 2009 to October 2009.
Lymphocyte separation medium (Tianjin, China); 20% fetal bovine serum (Gibco); 10 ng/mL vascular endothelial growth factor (PeproTech, Rochy Hill, New Jersey, USA); M199 medium (Gibco); fibronectin (BD Biosciences, Two Oak Park, Bedford, MA, USA); CD34 (BD Biosciences PharmingenTM, Bedford, MA, USA); CD133 (eBioscience. San Diego, USA, 1:100); CD105 (BD Biosciences PharmingenTM ); fluorescein isothiocyanate-conjugated secondary antibody (Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China, 1:50); 1,1'-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate labeled low density lipoprotein (Moleculor Probe, invitrogen Carlsbad, CA, USA); fluorescein isothiocyanate-labeled Ulex europaeus agglutinin 1 (10 μg/mL, Sigma, santa clara, CA, USA); flow cytometry (BD Calibur USA); inverted biological microscope (NIKON); CO2 incubator thermostat (Thermo Scientific infusafe); laser scanning confocal microscope (OLYMPUS); microscopic digital imaging system (OLYMPUS); ultrapure water purification system (Millipore); electronic balance (America).
Isolation of mononuclear cells
Sterile peripheral blood/umbilical cord blood (15 mL) was collected from healthy volunteers. Peripheral blood samples were from physical examinees in the Neurology Department, the First Hospital of Jilin University, and umbilical cord blood samples were from healthy pregnant women in the Department of Gynecology and Obstetrics, the First Hospital of Jilin University. Written informed consent was obtained from all subjects.
Mononuclear cells at 1×106/cm2 were placed in 24-well plate covered with fibronectin and immersed in M199 medium supplemented with 20% fetal bovine serum and 10 ng/mL vascular endothelial growth factor, followed by incubation in a 5% CO2 incubator at 100% humidity at 37 ℃. Early-stage endothelial progenitor cells: following 3 days of static culture, non-adherent cells were removed by washing, and the medium was changed every 3 days. Late-stage endothelial progenitor cells: following 24 hours of static culture, non-adherent cells were removed by washing, and half of the medium was changed every day for 7 days. The medium was subsequently changed as for the early-stage endothelial progenitor cells.
The numbers of cell colonies on the 24-well plate were counted and compared at 7 and 14 days after the start of cell culture. A cell mass was defined as being composed of more than 10 cells. Colonies in 11 visual fields were counted under a phase contrast microscope. Mean values were calculated based on the numbers of cells from three wells.
Identification of endothelial progenitor cells Growth characteristics of endothelial progenitor cells
Endothelial progenitor cells cultured at 0, 3, 7, 10 and 14 days were used for observation of cell morphology.
Adherent cells were cultured with 4% paraformaldehyde for 7 days and washed with phosphate-buffered saline. Reagent A (peroxidase inhibitor) was then added and cells were cultured for 20 minutes, followed by washing in phosphate-buffered saline. Reagent B (animal non-immune serum) was then added. Vascular endothelial growth factor receptor-2 (KDR) or CD31 first antibodies were added after 30 minutes. Phosphate-buffered saline was used in place of vascular endothelial growth factor receptor-2 or CD31 in the negative control group. It was known that positive blank films were used as positive controls. The cells were cultured at 4 ℃ overnight, followed by the addition of reagent C (biotin-labeled secondary antibody), washing in phosphate-buffered saline, and the addition of reagent D (streptavidin-peroxidase). Cells were stained with 3,3'-diaminobenzidine and counterstained with hematoxylin for observation under an inverted microscope. Brown color indicated positive staining, and the intensity of the brown staining was used to indicate the level of antigen expression.
Following 7 days in culture with 4% paraformaldehyde, adherent cells were washed with phosphate-buffered saline, and covered with serum. The first antibody (CD34 and CD133, concentration 1:100) was added, and cells were maintained at 4 ℃ overnight. Cells were then washed three times with phosphate-buffered saline, 5 minutes once, before addition of the fluorescein isothiocyanate-conjugated secondary antibody (1:50). They were incubated in the dark for
30 minutes, and washed a further three times with phosphate-buffered saline, 5 minutes once. Propidium iodide was used for nuclear staining, and the cells were maintained in the dark at 4 ℃ to prevent quenching. Cells were observed using an inverted fluorescence microscope.
The adherent cells were incubated with 10 ug/mL 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine-labeled-acetylated-low density lipoprotein at 37 ℃ for 4 hours, fixed with 2% paraformaldehyde for 10 minutes, and immersed in phosphate-buffered saline. Fluorescein isothiocyanate-Ulex europaeus agglutinin-1 (10 μg/mL) was added to the cells followed by incubation at 37 ℃ for 1 hour. Using confocal microscope, 1,1'-dioctadecyl-3,3,3',3'- tetramethylindocarbocyanine-labeled-acetylated-low density lipoprotein and fluorescein isothiocyanate-Ulex europaeus agglutinin 1 double-stained cells were identified as differentiating endothelial progenitor cells.
Primary cells were selected, adherent cells (2×105) were mixed with CD34, CD105, and CD133 monoclonal antibodies, and incubated at room temperature for 30 minutes. The cells were rinsed twice with phosphate-buffered saline, fluorescein isothiocyanate-labeled secondary antibody was added in the dark, and cells were maintained at 4 ℃ in the dark for 30 minutes, and then immersed in phosphate-buffered saline. Suspended cells in 300 uL phosphate-buffered saline containing were detected on the flow cytometry.
Morphological characteristics and identification of endothelial progenitor cells from human umbilical cord blood and peripheral blood.
Umbilical cord blood progenitor cells have stronger proliferative, differentiation and colony-forming abilities as well as a greater ability to produce autocrine growth factors than peripheral blood cells. Umbilical cord blood progenitor cell donation is safe, and peripheral blood samples are easy to obtain, with no concerns regarding immunorejection for autologous cells transfusion and vascular reconstruction using peripheral blood. Therefore, human umbilical cord and peripheral blood samples were selected for the present study. Factors influence the number of endothelial progenitor cells, including pathological factors and drugs. Age, gender, hypertension, coronary heart disease, diabetes, hypercholesterolemia, hyperhomocysteinemia, smoking and drinking which are all high risks for reducing the amount of endothelial progenitor cells in the peripheral blood and reducing the ability of angiogenesis. Estradiol influences endothelial progenitor cells mobilization because of menstrual cycle[9], stromal cells derived factor can promote endothelial progenitor cells homing and angiogenesis[10]. At the same time, endothelial progenitor cells can not only raise some genes expression, which plays an important role in angiogenesis, anti-arteriosclerosis, such as LL-37, pyruvate dehydrogenase lipoamide kinase isozyme 4, alpha-2-macroglobulin, growth differentiation factor 15, pigment epithelium-derived factor, lectin, galactoside-binding, soluble, 3[11]; but also raise platelet-derived growth factor receptor expression[12]. On acute stress state, endothelial progenitor cells in peripheral blood circulation are increasing, but it is that the number of apoptotic cells is also higher, the migration ability of endothelial progenitor cells is slower significant, and the ability for forming blood capillary nets to recovery nerve function is more difficult[13].
In 2000, the existence of two subtypes of endothelial progenitor cells was proposed: early and late endothelial progenitor cells. Early endothelial progenitor cells are derived from peripheral blood and are fusiform and bipolar needle-shaped cells that undergo colony-like aggregation after 4-7 days after primary culture. After 3-4 weeks, no further proliferation could be seen, and apoptosis occurred. Endothelial cell-specific surface marker expression is low, and the cells express some markers common to early endothelial progenitor cells, macrophages, and hematopoietic stem cells, such as CD14, CD45, and CD133. Late endothelial progenitor cells originate from the bone marrow, and show a typical cobblestone-like shape. These late endothelial progenitor cells proliferate steadily for more than 12 weeks, and express endothelial cell-specific antigens. These cells provide a better source of seed cells because of their proliferative characteristics. Two different approaches to the definition and identification of endothelial progenitor cells currently exist: one involves the use of cell surface markers for identification, which is the method most commonly reported in previous studies; the other method is based on the strong clonogenic ability of stem cells. Endothelial progenitor cells identified with the latter method are called endothelial outgrowth cells or late endothelial progenitor cells. We used two different culture methods, but found no significant differences in biological characteristics, cell morphologies, surface antigens, colony formation, or proliferative abilities between endothelial progenitor cells cultured using the two methods. These findings are inconsistent with the results of some other studies[21]. This discrepancy may be the result of different culture methods, and/or different sample sources and isolation methods.
1 探寻出了一条稳定实用的内皮祖细胞体外分离、培养和鉴定方法。 2 人脐带血和外周血中存在内皮祖细胞,浓度梯度离心联合贴壁筛选获得的单个核细胞在血管内皮生长因子的诱导培养下可分化成内皮祖细胞。
内皮祖细胞(endothelial progenitor cells,EPCs)是一类能够分化为内皮细胞的多潜能干细胞,存在于胎肝、脐血、骨髓及外周血中。机体受到损伤或缺血等打击时,EPCs动员、募集到损伤、缺血缺氧组织处,在血管损伤后内皮的修复及侧支循环血管网的建立方面发挥着重要的作用。研究表明,EPCs参与缺血性脑卒中后新生血管的形成、修复损伤的血管内皮、促进神经再生,促进侧支循环的建立,构建微血管组织工程网络等方面具有广泛的应用前景。 本实验直接采集临床患者外周血和脐带血作为标本,分离出内皮祖细胞,并对其生物学特性进行鉴定,为临床缺血性脑卒中患者EPCs的自体回输、内皮祖细胞库的建立提供了理论和技术支持。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||