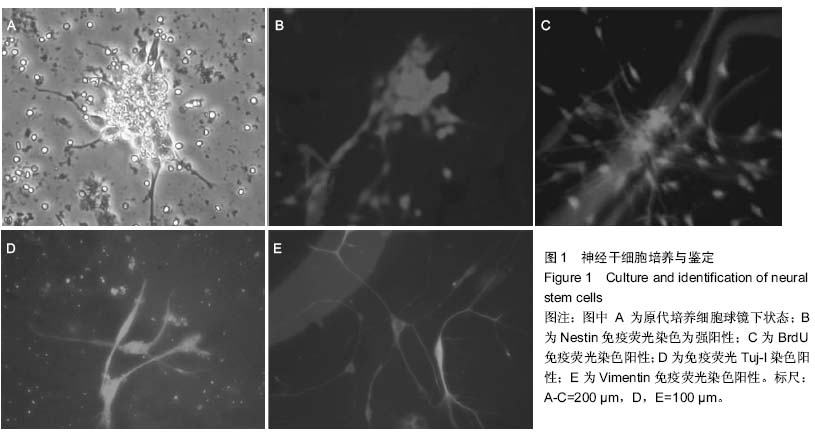
| [1] Ramasamy S,Narayanan G,Sankaran S, et al.Neural stem cell survival factors. Arch Biochem Biophys.2013; 534(1-2):71-87.
[2] Yang K,Han S,Shin Y, et al.A microfluidic array for quantitative analysis of human neural stem cell self-renewal and differentiation in three-dimensional hypoxic microenvironment. Biomaterials. 2013;34(28):6607-6614.
[3] 王子才,姜志梅.中国康复医学会第2届儿童康复学术会议、中国残疾人康复协会第9届小儿脑瘫康复学术会议暨国际交流会议[J].实用儿科临床杂志,.2006,21(24):1742.
[4] Piltti KM, Salazar DL, Uchida N,et al. Safety of human neural stem cell transplantation in chronic spinal cord injury.Stem Cells Transl Med.2013;2(12):961-974.
[5] Vargus-Adams JN, Martin LK, Maignan SH, et al. The GMFM,PEDI, and CP-QOL and perspectives on functioning from children with CP, parents, and medical professionals.J Pediatr Rehabil Med.2011;4(1):3-12.
[6] 施炳培,李惠,卜怀娣,等.穴位注射治疗小儿脑性瘫痪精细运动功能障碍ST例疗效观察[J].中国康复理论与实践,2006,12(2): 105-106.
[7] 李迎.脐血干细胞移植与神经生长因子联合治疗小儿脑性瘫痪的安全性分析[J].中国组织工程研究,2014,18(28):4588-4592.
[8] 闫冬梅,汪洪流,周培杰,等.人神经干细胞治疗小儿脑瘫的临床安全性研究[J].中国妇幼保健,2014,29(22):3595-3597.
[9] 钟占强,王黎明,周建军,等.脐带间充质干细胞治疗20例小儿脑瘫效果观察[J].中国综合临床,2012,28(10):1029-1031.
[10] 贺力男,孟祥琨,赵廷宝.脐带间充质干细胞治疗痉挛性脑瘫1例临床分析[J].医学信息,2012,25(11) 80-81.
[11] 杨静,滑蓉蓉,郑培,等.间充质干细胞移植治疗小儿脑瘫疗效分析[J].武警医学,2012,23(7):586-589,592.
[12] 杨华强,张荣环,杜玲,等.脐带间充质干细胞移植对脑瘫患儿运动功能的影响[J].现代生物医学进展,2012,12(2):250-252.
[13] 王亚莉,陈国军,方凤,等.自体转化神经干细胞治疗脑瘫合并顽固癫痫2例[J].临床荟萃, 2012,27(11):993-994.
[14] 吴景文,贾丹兵,薛超,等.干细胞移植治疗小儿脑瘫97例[J].中国中医药现代远程教育,2011,9(24):45-47.
[15] 冯梅,高红霞,代喜平,等.脐血干细胞移植治疗重症脑瘫30例临床疗效观察[J].中国输血杂志,2011,24(7):602-604.
[16] 王金刚,尹忠民,闻华,等.脐带间充质干细胞治疗脑瘫51例疗效观察[J].中国全科医学,2011,14(21):2446-2447.
[17] 贺声,栾佐,屈素清,等.超声引导脑室内神经干细胞移植治疗小儿脑瘫[J].中国介入影像与治疗学,2011,8(2):94-97.
[18] 李志刚,孙庆华,施艳萍.脐带源间充质干细胞脑室内移植治疗脑瘫的临床观察[J].医学信息(下旬刊),2011,24(10):100.
[19] 王晓东,杨静,李敏,等.骨髓间充质干细胞移植对小儿脑瘫患者粗大运动功能的影响[J].中日友好医院学报,2010,24(6):337-342.
[20] 邢利和,张丽欣,张丽丽,等.脐血干细胞联合神经生长因子和物理康复治疗小儿脑性瘫痪[J].中国组织工程研究,2012,16(41): 7777-7781.
[21] 刘岩松,王晓东,杨静,等.自体骨髓间充质干细胞移植脑性瘫痪儿童运动功能的评价[J].中国组织工程研究,2012,16(23): 4290- 4295.
[22] 陈文明,胡运新,尹靖宇,等.自体骨髓间充质干细胞回输对脑性瘫痪儿童运动功能发育的影响[J].中国组织工程研究与临床康复, 2011,15(40):7589-7592.
[23] 杜侃,栾佐,屈素清,等.骨髓间充质干细胞移植治疗小儿重度脑性瘫痪的疗效观察[J]. 临床儿科杂志,2011,29(1):55-58.
[24] 陶其强,唐明淇,许冬联,等.脐带血干细胞治疗小儿脑性瘫痪10例近期疗效分析[J]. 中外健康文摘,2009,6(35):18-19.
[25] 黄超,周盛年,付秀鑫,等.神经干细胞移植治疗脑性瘫痪的疗效观察[J].中国医疗前沿,2009,4(2):1-2.
[26] 吴景文,贾丹兵,曲超法,等.干细胞移植治疗小儿脑瘫的临床疗效:40例报告[J].中华神经外科疾病研究杂志,2011,10(5):424- 427.
[27] De Feo D, Merlini A, Laterza C,et al.Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection.Curr Opin Neurol. 2012;25(3): 322-333.
[28] 张丽欣,邢利和,张丽丽,等.脐血干细胞治疗小儿脑瘫的临床安全性研究[J].中国全科医学,2010,13(17):1868-1870.
[29] Russell DJ,Palisano RJ,Walter S, et al. Evalrating motor function in children with down syndrome:validity of the GMFM.Dev Med Child Neurol.1998; 40(10):693-701.
[30] Provost B, Crowe TK, McClain C.Concurrent validity of the Bayley Scales of Infant Development II Motor Scale and the Peabody Developmental Motor Scales in two-year-old children.Phys Occup Ther Pediatr.2000;20(1):5-18. |