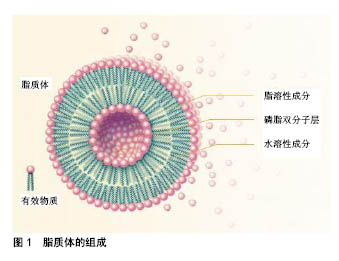
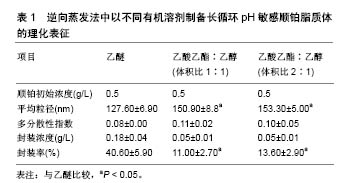
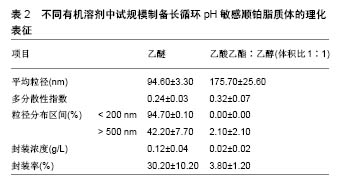
| [1] Zeb A,Qureshi OS,Kim HS,et al. High payload itraconazole-incorporated lipid nanoparticles with modulated release property for oral and parenteral administration. J Pharm Pharmacol. 2017;69(8):955-966. [2] Qureshi OS,Kim HS, Zeb A,et al. Sustained release docetaxel- incorporated lipid nanoparticles with improved pHarmacokinetics for oral and parenteral administration. J Microencapsul. 2017;34(3): 250-261. [3] Battaglia L,Gallarate M,Peira E,et al. Solid lipid nanoparticles for potential doxorubicin delivery in glioblastoma treatment: preliminary in vitro studies. J Pharm Sci. 2014;103(7):2157-2165. [4] Kakkar D,Dumoga S,Kumar R,et al. PEGylated solid lipid nanoparticles: design, methotrexate loading and biological evaluation in animal models. Med Chem Commun. 2015;6(8):1452-1463. [5] Patel MN,Lakkadwala S,Majrad MS,et al. Characterization and evaluation of 5-fluorouracil-loaded solid lipid nanoparticles prepared via a temperature-modulated solidification technique. AAPS Pharm Sci Tech. 2014;15(6):1498-1508. [6] Lee WH,Loo CY,Traini D,et al. Nano-and micro-based inhaled drug delivery systems for targeting alveolar macropHages. Expert Opin Drug Deliv. 2015;12(6):1009-1026. [7] Giuberti CDS,Boratto FA,Degobert G,et al. Investigation of alternative organic solvents and methods for the preparation of long-circulating and pH-sensitive liposomes containing cisplatin. J Liposome Res. 2013;23(3):220-227. [8] Leite EA,Souza CM,Carvalho-Júnior AD,et al.Encapsulation of cisplatin in long-circulating and pH-sensitive liposomes improves its antitumor effect and reduces acute toxicity.Int J Nanomedicine. 2012; 7:5259-5269.[9] Giuberti CS,Reis ECO,Rocha TGR,et al. Study of the pilot production process of long-circulating and pH-sensitive liposomes containing cisplatin. J Liposome Res. 2011;21:60-69. [10] Biswas S, Kumari P, Lakhani PM, Ghosh B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J PHarm Sci. 2016;83:184-202. [11] Gothwal A,Khan I,Gupta U.Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharm Res. 2016;33(1):18-39. [12] Ng CM,Loh HS,Muthoosamy K,et al. Conjugation of insulin onto the sidewalls of single-walled carbon nanotubes through functionalization and diimide-activated amidation. Int J Nanomedicine. 2016;11: 1607-1614. [13] Johnsson M, Edwards K. Liposomes, disks and spHerical micelles: aggregate structure in mixtures of gel pHase pHospHatidylcholines and poly(ethyleneglycol)-pHospHolipids. BiopHys J. 2003;85: 3839-3847. [14] Kasper JC, Friess W. The freezing step in lyopHilization: pHysico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopHarmaceuticals. Eur J Pharm Biopharm. 2011;78:248-263. [15] Leite EA,Lana AMQ, Junior ADC, et al.Acute toxicity study of cisplatin loaded long-circulating and pH-sensitive liposomes administered in mice. J Biomed Nanotechnol. 2012;8:229-239. [16] Prabhakar N, Zhang J,Desai D,et al. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int J Nanomedicine. 2016;11:6591-6608. [17] Wagner A,Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011: 591325. [18] Wessman P, Edwards K, Mahlin D.Structural effects caused by spray- and freeze-drying of liposomes and bilayes disks. J PHarm Sci. 2010; 99:2032-2048. [19] Desai D, Zhang J, Sandholm J, et al. Lipid bilayer-gated mesoporous silica nanocarriers for tumor-targeted delivery of zoledronic acid in vivo. Mol Pharm. 2017;14(9):3218-3227. [20] Han N,Zhao Q,Wan L,et al. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl Mater Interfaces. 2015;7(5):3342-3351. [21] Ganesanab P, Narayanasamya D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm 2017;6:37-56. [22] Geszke-Moritz M, Moritz M.Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies.Mater Sci Eng C Mater Biol Appl. 2016;68:982-994.[23] Charcosset C, Audrey J, Valour JP, et al. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem Eng Res Des. 2015;94(2):508-515. [24] Huang Z, Li X, Zhang T, et al. Progress involving new techniques for liposome preparation. Asian J Pharm Sci. 2014;9(4):176-182. [25] Wagner A,Vorauer-Uhl K,Katinger H.Liposomes produced in a pilot scale: production, purification and efficiency aspects.Eur J Pharm Biopharm. 2002;54(2):213-219. [26] Pham TT, Jaafar-Maalej C,Charcosset C,et al.Liposome and niosome preparation using a membrane contactor for scale-up.Colloids Surf B Biointerfaces. 2012;94:15-21. [27] Silva JO, Fernandes RS,Lopes SCA,et al. pH-Sensitive, Long-Circulating Liposomes as an Alternative Tool to Deliver Doxorubicin into Tumors: a Feasibility Animal Study. Molecular Imaging Biol.2016;18(6):1-7. [28] Carlesso FN, Araújo RS, Fuscaldi LL, et al. Preliminary data of the antipancreatic tumor efficacy and toxicity of long-circulating and pH-sensitive liposomes containing cisplatin. Nucl Med Commun. 2016;37(7):727-734. [29] Abuasal BS,Lucas C,Peyton B,et al.Enhancement of intestinal permeability utilizing solid lipid nanoparticles increases [gamma]-tocotrienol oral bioavailability. Lipids. 2012;5:461-469. [30] Andrade ME, Araujo RS,de Barros PA,et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr.2015;34:1080-1087. [31] Araujo JG, Mota L, Leite EA, et al. Biodistribution and antitumoral effect of long-circulating and pH sensitive liposomal cisplatin administered in Ehrlich tumor-bearing mice. Exp Biol Med(Maywood). 2011;236:808-815. [32] Arifa RD,Madeira MF, de Paula TP,et al. Inflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1beta and IL-18 in mice. Am J Pathol. 2014;184: 2023-2034. [33] Arivarasu NA, Fatima S,Mahmood R. Effect of cisplatin on brush border membrane enzymes and anti-oxidant system of rat intestine. Life Sci. 2007;81:393-398. [34] Atista MA, Nicoli JR, Martins Fdos S,et al. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. JPEN J Parenter Enteral Nutr. 2012;36:69-76. [35] Bearcroft CP, Domizio P, Mourad FH,et al. Cisplatin impairs fluid and electrolyte absorption in rat small intestine: a role for 5-hydroxytryptamine. Gut.1999;44:174-179. [36] Beutheu Youmba S , Belmonte L , Galas L , et al. Methotrexate modulates tight junctions through NF-kappaB, MEK, and JNK pathways. J Pediatr Gastroenterol Nutr. 2012;54:463-470. [37] Beutheu S,Ouelaa W,Guerin C, et al. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin Nutr. 2014;33:694-701. [38] Bodiga VL, Bodiga S,Surampudi S, et al. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition. 2012;28:572-580. [39] Carvalho Junior AD,Vieira FP, Melo VJ, et al. Preparation and cytotoxicity of cisplatin-containing liposomes. Braz. J Med Biol Res. 2007;40:1149-1157. [40] de Carvalho Maroni L, de Oliveira Silveira AC, Leite EA, et al. Antitumor effectiveness and toxicity of cisplatin-loaded long-circulating and pH-sensitive liposomes against Ehrlich ascitic tumor. Exp Biol Med (Maywood). 2012;237:973-984. [41] Dobrovolskaia MA,McNeil SE.Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469-478. [42] Elsabahy M , Wooley KL. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem Soc Rev. 2013;12:5552-5576. [43] Ferreira Ddos S,Lopes SC,Franco MS, et al. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013;4:1099-1123. [44] Frankenberger M,Haussinger K,Ziegler-Heitbrock L. Liposomal methylprednisolone differentially regulates the expression of TNF and IL-10 in human alveolar macropHages. Int ImmunopHarmacol. 2015; 5:289-299. [45] Araújo RS, Silveira ALM, de Sales E Souza ÉL, et al. Intestinal toxicity evaluation of long-circulating and pH-sensitive liposomes loaded with cisplatin. Eur J Pharm Sci. 2017;106:142-151. [46] Monteiro LOF,Fernandes RS,Oda CMR,et al. Paclitaxel-loaded folate-coated long circulating and pH-sensitive liposomes as a potential drug delivery system: A biodistribution study. Biomed Pharmacother. 2018;97:489-495. [47] Gunji S,Obama K,Matsui M,et al. A novel drug delivery system of intraperitoneal chemotherapy for peritoneal carcinomatosis using gelatin microspHeres incorporating cisplatin.Surgery. 2013;154:991-999. [48] Duan Y,Wei L,Petryk J.Formulation, characterization and tissue distribution of a novel pH-sensitive long-circulating liposome-based theranostic suitable for molecular imaging and drug delivery. Int J Nanomedicine. 2016;11:5697-5708. [49] Leite EA,Giuberti Cdos S,Wainstein AJ, et al. Acute toxicity of long-circulating and pH-sensitive liposomes containing cisplatin in mice after intraperitoneal administration. Life Sci. 2009;8(19-20): 641-649. [50] Schillaci O, Corleto VD, Annibale B,et al. Single photon emission computed tomography procedure improves accuracy of somatostatin receptor scintigraphy in gastro-entero pancreatic tumours. Ital J Gastroenterol Hepatol.1999;31 Suppl 2:S186-189. [51] Li J,Sun K,Ni L,et al. Sodium selenosulfate at an innocuous dose markedly prevents cisplatin-induced gastrointestinal toxicity. Toxicol Appl PHarmacol. 2012;258:376-383. [52] Nose S,Wasa M,Tazuke Y,et al.Cisplatin upregulates glutamine transport in human intestinal epithelial cells: the protective mechanism of glutamine on intestinal mucosa after chemotherapy. JPEN J Parenter Enteral Nutr. 2010;34:530-537. [53] Kohli AG, Kieler-Ferguson HM, Chan D, et al. A robust and quantitative method for tracking liposome contents after intravenous administration.J Control Release. 2014;176:86-93. [54] Kohli AG, Kierstead PH, Venditto VJ, et al. Designer lipids for drug delivery: from heads to tails. J Control Release.2014;90:274-287. [55] Lajunen,T, Kontturi LS,Viitala L,et al. Indocyanine green-loaded liposomes for light-triggered drug release. Mol Pharm. 2016;13: 2095-2107. |
.jpg)
.jpg)