| [1]Rajaii F, McCoy AN, Smith TJ. Cytokines are both villains and potential therapeutic targets in thyroid-associated ophthalmopathy: From bench to bedside. Expert Rev Ophthalmol.2014;9(3):227-234.[2]Jang SY, Lee KH, Oh JR, et al. Development of Thyroid-Associated Ophthalmopathy in Patients Who Underwent Total Thyroidectomy. Yonsei Med J. 2015;56(5):1389-1394. [3]Salvi M. Immunotherapy for Graves' ophthalmopathy. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):409-414. [4]Kavoussi SC, Giacometti JN, Servat JJ, et al. The relationship between sex and symmetry in thyroid eye disease. Clin Ophthalmol. 2014;8:1295-1300. [5]居红格,谢建兰,郭新建,等.眼结膜粘膜相关淋巴组织边缘带B细胞淋巴瘤临床病理分析[J].临床与实验病理学杂志, 2014,30(8):840-843. [6]张庆,邵毅,关晏星,等.标准剂量泼尼松联合谷氨酰胺对I131治疗后Graves眼病的保护作用[J].重庆医学, 2017,46(13):1775-1778.[7]张袆,张险峰,韩辉,等.中小剂量甲泼尼龙联合甲氨蝶呤与硒酵母对Graves眼病的疗效观察[J].中华内分泌代谢杂志, 2016,32(1):24-26.[8]唐宇光.甲巯咪唑联合左甲状腺素治疗甲亢性突眼的临床效果分析[J].黑龙江医药,2016,29(2):279-280.[9]张道武.醋酸泼尼松龙联合环磷酰胺治疗甲亢突眼的疗效观察[J].医学信息,2014(36):261.[10]张楠,卢弘.环孢素A在非感染性葡萄膜炎治疗中的应用[J].眼科新进展, 2017,37(3):293-297.[11]李敏,崔雪娇,李玉铢,等.Graves眼病患者生活质量及眼病特异性生活质量的相关性[J].中国医科大学学报,2016,45(5):445-447,451.[12]俞丹洋,魏锐利,李玉珍,等.儿童及青少年Graves眼病的临床特点及疗效分析[J].中国实验眼科杂志,2016,34(8):716-719.[13]Di Tommaso C, Valamanesh F, Miller F, et al. A novel cyclosporin a aqueous formulation for dry eye treatment: in vitro and in vivo evaluation. Invest Ophthalmol Vis Sci. 2012;53(4):2292-2299. [14]Junghanss C, Rathsack S, Wacke R, et al. Everolimus in combination with cyclosporin a as pre- and posttransplantation immunosuppressive therapy in nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(7):1061-1068. [15]Afonso M, Perrotti V, Rapani M, et al. Immunoexpression of angiogenesis and proliferation markers in patients treated with cyclosporin A. Minerva Stomatol. 2014;63(3):59-67.[16]Kim SY, Shim MS, Kim KY, et al. Inhibition of cyclophilin D by cyclosporin A promotes retinal ganglion cell survival by preventing mitochondrial alteration in ischemic injury. Cell Death Dis. 2014;5:e1105. [17]Fuhrmann A, Lopes P, Sereno J, et al. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model. Biochem Pharmacol. 2014;88(2):216-228.[18]Cheng M. Hartmann Stahelin (1925-2011) and the contested history of cyclosporin A. Clin Transplant. 2013;27(3):326-329. [19]Mori M, Inoue D, Arima H, et al. Therapeutic efficacy of cyclosporin A for refractory angioimmunoblastic T cell lymphoma. Rinsho Ketsueki. 2010;51(5):332-338.[20]Liu H, Wang Y, Han F, et al. Gelatin-stabilised microemulsion-based organogels facilitates percutaneous penetration of Cyclosporin A in vitro and dermal pharmacokinetics in vivo. J Pharm Sci. 2007;96(11): 3000-3009.[21]刘洪卓.环孢素A皮肤给药系统的设计与评价[D].沈阳:沈阳药科大学,2007.[22]王黎.中药巴布膏剂的研究概况[J].中华实用中西医杂志, 2007,20(17): 1544-1546.[23]王雪,高建德.中药巴布剂的研究进展[J].中医儿科杂志, 2007,3(2):54.[24]庞武耀,李明亚,谢清春.巴布剂的研究现状[J].亚太传统医药, 2008,4(12): 133-136.[25]宋文娟.接骨巴布膏的制备及临床应用[J].时珍国医国药, 2006,17(11): 2236-2237.[26]朱庆文,李海燕,杜红.中药经皮给药物理促透技术与发展思路研究[J].国际生物医学工程杂志,2015,38(4):253-256.[27]冯金鹰.蟾酥巴布膏的研究[D].济南:山东中医药大学,2010.[28]国家药典委员会.中华人民共和国药典:2000年版.一部[M].北京:化学工业出版社,2000.[29]刘方艺.经络贴巴布剂的制备工艺与体外经皮渗透研究[D].广州:广州中医药大学,2011.[30]30梁媛媛,马琳,崔黎丽,等.正极性驻极体和氮酮对环孢素A的体外透皮促渗作用[J].第二军医大学学报,2012,33(6):617-620.[31]王岚,刘淑芝,王彦礼,等.萸连巴布膏抗消化性溃疡的药效学研究[J].中国实验方剂学杂志,2011,17(7):194-197.[32]戎宽.肿痛消巴布膏对LDH大鼠TNF-a?IL-1影响的实验研究[D].长沙:湖南中医药大学,2014.[33]望开鹏.环孢素A纳米粒丝素蛋白-聚乙烯醇共混膜剂的研究[D].苏州:苏州大学,2011.[34]刘先继,王永强,刘先锋,等.乳癖消巴布膏制备工艺[J].实用中医内科杂志, 2014,28(8):126-127.[35]徐晖,王绍宁,谷野,等.巴布膏剂研制的一些问题[J].中医外治杂志, 2005, 14(6):3-4.[36]庞丹梅.万灵五香巴布膏的研制[D].杭州:浙江大学,2005.[37]Domingo-Chiva E, Ruíz-Ramos J, Marqués-Miñana MR, et al. Drug Interaction Between Oral Cyclosporine Modified and Iron. Ann Pharmacother. 2014;48(7):932-935.[38]杜茂波.萸连巴布膏的药学研究与评价[D].北京:中国中医科学院,2009.[39]张韻慧,王春杰,晋兴华,等.吲哚美辛水凝胶贴剂小鼠体外透皮性能研究[J].现代生物医学进展,2013,13(34):6619-6622,6706.[40]杜丽娜,金义光.经皮给药系统研究进展[J].国际药学研究杂志, 2013, 40(4):379-385.[41]王海东,倪善红,孙增先.液相色谱-质谱联用和酶增强免疫分析法测定人全血环孢素A浓度比较[J].医药导报, 2016,35(10):1064-1068. [42]黄培池,王娟.HPLC法测定环孢素滴眼液中环孢素的含量[J].淮海医药, 2015,33(4):382-383.[43]沙波,齐永贵,叶启发,等.肝肠联合移植后的免疫抑制治疗体会[J].中国器官移植杂志,2000,21(5):283-284. [44]袁成,王景祥,贾暖,等.兔角膜环孢素A含量测定及其经时过程[J].中国临床药学杂志,1998,7(6):287-290. [45]周晓庆,张秀华,黄学孙,等.环孢素A滴眼液的制备及治疗控制[J].中国医院药学杂志,2005,25(6):574-575.[46]王延东,陈茂玲,叶成添,等.环孢素眼用乳剂的制备及质量控制[J].中国实用医药,2010,12 (5):7-9.[47]陈洁,杨利平,刘春雨,等.高效液相色谱法测定环孢素滴眼液中环孢素的含量[J].药物分析杂志,2006,26(3):372-373. [48]章元沛.药理学实验[M].2版.北京:人民卫生出版社,1996:222. [49]胡楠楠,王雪峰,赵雪,等.敷胸巴布贴制剂对健康家兔皮肤刺激实验研究[J].辽宁中医杂志,2012,39(6):1168-1169.[50]王秋菊,吕娟,张予阳,等.乳癖消巴布膏的刺激性?过敏性及经皮毒性研究[J].药学实践杂志,2008,26(1):31-34.[51]Garcovich S, Garcovich C, Cassano N, et al. Cyclosporine A microemulsion in dermatological practice: is oral solution better tolerated than soft gelatin capsules? G Ital Dermatol Venereol. 2015;150(4):473-474.[52]Wendlow SH, Campa E, Harris NL. WHO Classification Tumors of the Haematopoietic and Lymphoid Tissues. Lyon:IARC, Press. 2008:214. [53]薛彬,张晴,郑占伟,等.HPLC法同时测定环孢素A和其衍生物的含量[J].化学与生物工程,2016,33(3):68-70.[54]Fruši?-Zlotkin M, Soroka Y, Tivony R, et al. Penetration and biological effects of topically applied cyclosporin A nanoparticles in a human skin organ culture inflammatory model. Exp Dermatol. 2012;21(12): 938-943. |
.jpg)
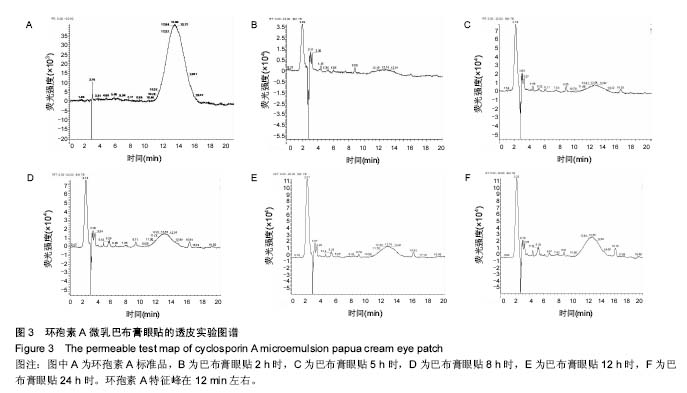

.jpg)
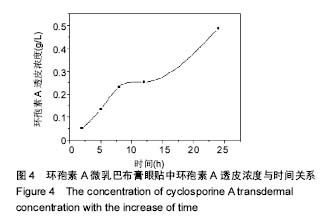
.jpg)
.jpg)