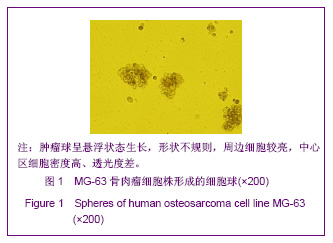
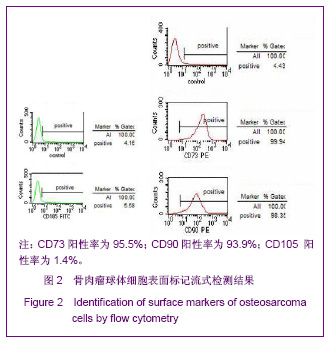
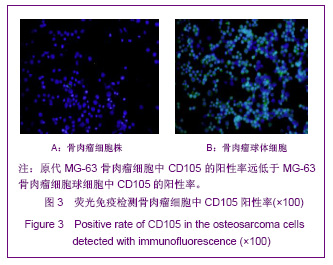
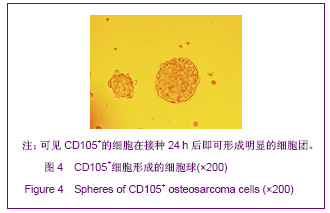
同类文献比较
1、
TI:Identification and gene expression profiling of tumor-initiating cells isolated from human osteosarcoma cell lines in an orthotopic mouse model.
AU:Rainusso N, Man TK, Lau CC, Hicks J, Shen JJ, Yu A, Wang LL, Rosen JM.
AD: Texas Children's Cancer Center and Hematology Service, Houston, USA.
SO: Cancer Biol Ther. 2011 Aug 15;12(4):278-287.
AB: In the cancer stem cell model a cell hierarchy has been suggested as an explanation for intratumoral heterogeneity and tumor formation is thought to be driven by this tumor cell subpopulation. The identification of cancer stem cells in osteosarcoma (OS) and the biological processes dysregulated in this cell subpopulation, also known as tumor-initiating cells (TICs), may provide new therapeutic targets. The goal of this study, therefore, was to identify and characterize the gene expression profiles of TICs isolated from human OS cell lines. We analyzed the self-renewal capacity of OS cell lines and primary OS tumors based upon their ability to form sphere-like structures (sarcospheres) under serum-starving conditions. TICs were identify from OS cell lines using the long-term label retention dye PKH26. OS TICs and the bulk of tumor cells were isolated and used to assess their ability to initiate tumors in NOD/SCID mice. Gene expression profiles of OS TICs were obtained from fresh orthotopic tumor samples. We observed that increased sarcosphere efficiency correlated with an enhanced tumorigenic potential in OS. PKH26Hi cells were shown to constitute OS TICs based upon their capacity to form more sarcospheres, as well as to generate both primary bone tumors and lung metastases efficiently in NOD/SCID mice. Genomic profiling of OS TICs revealed that both bone development and cell migration processes were dysregulated in this tumor cell subpopulation. PKH26 labeling represents a valuable tool to identify OS TICs and gene expression analysis of this tumor cell compartment may identify potential therapeutic targets.
2、
TI:Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo.
AU:Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G.
AD: Department of Experimental Medicine, Section of Histology and Embryology, 5 via L. Armanni, Second University of Naples, 80138 Naples, Italy.
SO: FASEB J. 2011 Jun;25(6):2022-30.
AB: This study aimed to identify, isolate, and characterize cancer stem cells from human primary sarcomas. We performed cytometric analyses for stemness and differentiation antigens, including CD29, CD34, CD44, CD90, CD117, and CD133, on 21 human primary sarcomas on the day of surgery. From sarcoma biopsies, we obtained 2 chondrosarcoma-stabilized cell lines and 2 osteosarcoma stabilized cell lines, on which sphere formation, side population profile, stemness gene expression, and in vivo and in vitro assays were performed. All samples expressed the CD133, CD44, and CD29 markers. Therefore, we selected a CD133(+) subpopulation from stabilized cell lines that displayed the capacity to grow as sarcospheres able to initiate and sustain tumor growth in nonobese diabetic/severe combined (NOD/SCID) mice, to express stemness genes, including OCT3/4, Nanog, Sox2, and Nestin, and to differentiate into mesenchymal lineages, such as osteoblasts and adipocytes. Our findings show the existence of cancer stem cells in human primary bone sarcomas and highlight CD133 as a pivotal marker for identification of these cells. This may be of primary importance in the development of new therapeutic strategies and new prognostic procedures against these highly aggressive and metastatic tumors.
3、
TI:BMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99-1 cell line.
AU:Wang L, Park P, Zhang H, La Marca F, Claeson A, Valdivia J, Lin CY.
AD: Spine Research Laboratory, Department of Neurosurgery, University of Michigan Medical School, Ann Arbor, USA.
SO: Cancer Biol Ther. 2011 Mar 1;11(5):457-63. Epub 2011 Mar 1.
AB: Previously, based on high ALDH activity, we showed that cancer stem cells (CSCs) could be identified as ALDH(br) cells from an aggressive human osteosarcoma OS99-1 cell line. In this study, we evaluate the impact of BMP-2 on CSCs.Three types of BMP receptors were expressed in freshly sorted ALDH(br) cells. In vitro, growth of the sorted ALDH(br) cells was inhibited by BMP-2. Using RT-PCR analysis, BMP-2 was found to down-regulate the expression of embryonic stem cell markers Oct3/4, Nanog, and Sox-2, and up-regulate the transcription of osteogenic markers Runx-2 and Collagen Type I. In vivo, all animals receiving ALDH(br) cells treated with BMP-2 did not form significant tumors, while untreated ALDH(br) cells developed large tumor masses in NOD/SCID mice. Immunostaining confirmed few Ki-67 positive cells were present in the sections of tumor containing ALDH(br) cells treated with BMP-2. These results suggest that BMP-2 suppresses tumor growth by reducing the gene expression of tumorigenic factors and inducing the differentiation of CSCs in osteosarcoma. BMP-2 or BMP-2-mimetic drugs, if properly delivered to tumor and combined with traditional therapies, may therefore provide a new therapeutic option for treatment of osteosarcoma.
4、
标题:应用低浓度长春新碱分离并鉴定MG63细胞系中的骨肉瘤干细胞
作者:娄楠(1),王岩(2),赵建武(1),高忠礼(1)
单位:(1)吉林大学中日联谊医院骨科。(2)吉林大学中日联谊医院临床基因诊疗中心。
摘要:目的 分离并鉴定人成骨肉瘤细胞系MG63中的骨肉瘤类肿瘤干细胞。方法 通过无血清培养基加入低浓度长春新碱的方法悬浮培养成骨肉瘤细胞球来进行分离和类干细胞富集。后行细胞形态学观察、细胞球免疫荧光染色、RTPCR、流式细胞仪鉴定细胞表面标志物,成骨、成脂肪诱导及裸鼠成瘤的一系列鉴定试验。结果 悬浮培养得到肉瘤细胞球,可持续传代细胞亚系“MG63M”,多能干细胞、胚胎干细胞标志物及多药耐药基因表达均为阳性。具有很强的自我更新及多项分化能力。结论 MG63细胞系中存在具有自我更新及多向分化能力的骨肉瘤干细胞,而采用神经干细胞培养基中添加低浓度长春新碱是从骨肉瘤细胞系中分离培养出骨肉瘤干细胞的有效办法。
出处:中国老年学杂志 2010;30(5):645-647
5、
标题:简化无血清培养及分离U2-OS人骨肉瘤细胞系肿瘤干细胞
作者:郭洪章(1),王栓科(2),杨忠义(1),欧阳振(3)
单位:(1)四川省攀枝花市第二人民医院骨科, (2)兰州大学第二医院骨科, (3)汉中3201医院骨科。
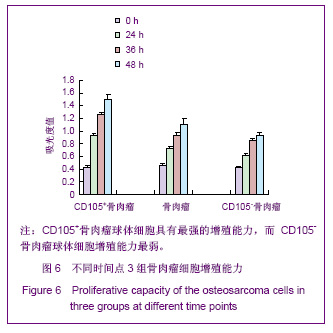
摘要:背景:已有实验应用成分复杂的无血清培养基从骨肉瘤组织中分离出干细胞样肿瘤细胞。目的:应用简化的无血清培养基在U2-OS人骨肉瘤细胞系中分离培养出骨肉瘤干细胞并对其作肿瘤干细胞的相关检验。设计、时间及地点:干细胞体外培养实验,于2006-02/2007-02在兰州大学第二医院骨科研究所完成。材料:U2-OS人骨肉瘤细胞系购自中科院上海生命科学研究院细胞库。无血清培养基为在DMEM/F12培养基中添加重组表皮生长因子(20μg/L)和重组成纤维生长因子(20μg/L),L-谷氨酰胺(2mmol/L),胰岛素(4U/L),青霉素G(1×10^5U/L),链霉素(100mg/L)。方法:取在含血清培养基中贴壁生长的U2-OS细胞,接种于含有表皮生长因子和成纤维生长因子的简化无血清培养基中,形成悬浮生长的骨肉瘤细胞球后观察其增殖、分化能力。用免疫细胞化学染色法检测间充质干细胞标志CD44、CD105蛋白在骨肉瘤细胞球中的表达。反转录-聚合酶链反应法检测U2-OS细胞及其肿瘤干细胞中胚胎多能干细胞管家基因Oct3/4mRNA的表达。主要观察指标:①悬浮细胞球的形成。②肿瘤干细胞增殖活性。③肿瘤干细胞的侵袭能力。④CD44、CD105蛋白在骨肉瘤细胞球细胞中的表达。⑤肿瘤干细胞中Oct3/4mRNA的表达。结果:U2-OS人骨肉瘤细胞系中有极少数的细胞能在简化无血清培养基中增殖形成骨肉瘤细胞球,具有很强的增殖分化能力,其表面具有间充质干细胞的膜分子CD44、CD105,表达胚胎干细胞管家基因Oct3/4mRNA。结论:U2-OS细胞系中存在肿瘤干细胞,简化无血清培养基可用于分离培养骨肉瘤干细胞。
出处:中国组织工程研究与临床康复 2009; 13(19): 3623-3627
6、
标题:人骨肉瘤细胞株U2-OS中分离并鉴定肿瘤干细胞
作者:欧阳振,王栓科,康学文,王翠芳,汪静,郭洪章
单位:兰州大学第二医院骨科研究所
摘要:目的:采用无血清培养法从人骨肉瘤细胞株U2-OS中分离肿瘤干细胞,检测肿瘤干细胞特异性标志物Stro-1的表达。方法:实验于2006-02/2007-02在兰州大学第二医院骨科研究所完成。①实验材料:人骨肉瘤细胞株U2-OS由中国科学院细胞库提供;DMEM/F12(1∶1)培养基(Gibco公司);胎牛血清(杭州四季青公司);PCR引物由上海生物工程公司合成。②实验方法:取原代培养的骨肉瘤细胞经胰蛋白酶消化制备单细胞悬液,用含表皮生长因子20μg/L、碱性成纤维细胞生长因子20μg/L、L-谷氨酰胺2mmol/L、胰岛素4U/L、青霉素100U/mL和链霉素100U/mL,pH7.2~7.5的无血清DMEM/F12培养基重悬细胞,常规培养5~7d,待培养基中悬浮的肉瘤细胞球体积较大后,用无血清培养基重新吹打成单细胞悬液,按1∶2或1∶3比例传代。③实验评估:从上述骨肉瘤细胞株中分离出的肿瘤细胞球,用MTT法检测其增殖能力,免疫磁珠分选Stro-1阳性细胞,反转录-聚合酶链法检测Stro-1阳性细胞中Stro-1 mRNA的表达,免疫细胞化学染色的方法检测分化后成骨细胞特异性抗原的表达。结果:①肿瘤干细胞增殖活性:增殖潜伏期约为24h,传代后1~2d即可见肿瘤干细胞形成,以后体积逐渐增大。第7天吸光度值最高,与0d吸光度值比较差异有显著性意义(P0.01)。②免疫磁珠法分选Stro-1阳性细胞:分选的Stro-1+细胞接种于无血清培养基后,24~48h即可形成和原代肿瘤干细胞球形态一样的干细胞球,而Stro-1-细胞却不能形成肿瘤细胞球。③骨肉瘤干细胞中Stro-1mRNA的表达:Stro-1+细胞和Stro-1-细胞mRNA扩增产物的吸光度值二者比较差异有显著性意义(P0.05)。④肿瘤干细胞分化:分化2周的肿瘤干细胞骨形态蛋白及Ⅰ型胶原酶均呈阳性表达。结论:骨肉瘤细胞株中存在骨肉瘤干细胞,并具有自我更新和多向分化的能力。
出处:中国组织工程研究与临床康复 2007; 11(24):4706-4709
7、
标题:Nanog mRNA在骨肉瘤类肿瘤干细胞中的表达和意义
作者:韩兴文,王栓科,康学文,王翠芳,何晶晶
单位:兰州大学第二医院骨科研究所
摘要:目的:观察骨肉瘤类肿瘤干细胞中Nanog基因的表达和意义。 方法:用无血清培养法从骨肉瘤细胞株U2-OS、MG-63中分离培养悬浮细胞球,免疫磁珠分选Stro-1阳性细胞,反转录-聚合酶链反应(RT-PCR)法检测Stro-1阳性细胞、Stro-1阴性细胞及悬浮生长细胞中Nanog mRNA的表达。 结果:Stro-1阳性细胞和悬浮细胞Nanog mRNA表达水平1.69±0.14,1.56±0.18,均高于Stro-1阴性细胞的表达水平0.19±0.35(P<0.05)。随着培养时间的延长悬浮细胞球Nanog mRNA的表达比早期悬浮细胞球有所下降(P<0.05)。 结论:骨肉瘤类肿瘤干细胞高度表达Nanog mRNA,该基因可能有助于骨肉瘤类肿瘤干细胞自我更新和维持其未分化状态。
出处:第四军医大学学报 2009;30(6):513-516
8、
标题:三维无血清条件培养结合抗癌药物分离人骨肉瘤肿瘤干细胞
作者:周松,李锋,肖骏,熊伟, 方忠,陈文坚,牛鹏彦
单位:华中科技大学同济医学院附属同济医院骨科
摘要:目的探讨无血清三维条件培养结合抗癌药物分离及鉴定人骨肉瘤肿瘤干细胞的可行性。方法将来源于人体的骨肉瘤细胞种植于1.2%藻酸盐凝胶中,并置于添加有阿霉素(Epirubicin,0.8μg/ml)的无血清DMEM/F12条件培养基中培养。培养7~10天后,可见凝胶内出现由单细胞增殖形成的单克隆球。取出该单克隆球,并通过细胞免疫荧光(Oct3/4和Nanog)、体内致瘤实验,检测该单克隆球细胞的生物特性。结果单克隆球主要由Oct3/4和Nanog阳性细胞组成,这些阳性细胞具有明显的致瘤作用。结论分离所得的单克隆细胞既能表达部分干细胞基因(Oct3/4和Nanog),又表现出明显的抗肿瘤药物性和体内致瘤性,三维无血清条件培养结合抗癌药物分离所得的单克隆骨肉瘤细胞可能为人骨肉瘤干细胞。
出处:实用肿瘤杂志 2010;(1)5-8