中国组织工程研究 ›› 2017, Vol. 21 ›› Issue (16): 2534-2539.doi: 10.3969/j.issn.2095-4344.2017.16.013
• 血管组织构建 vascular tissue construction • 上一篇 下一篇
C促红细胞生成素在脑梗死后微循环再通中的作用
张起顺,陈 勇,王朝辉,吴春芳,赵 俊
- 河南大学淮河医院神经内科,河南省开封市 475000
Carbamylated erythropoietin promotes vascular microcirculation following cerebral infarction
Zhang Qi-shun, Chen Yong, Wang Zhao-hui, Wu Chun-fang, Zhao Jun
- Department of Neurology, Huaihe Hospital of Henan University, Kaifeng 475000, Henan Province, China
摘要:
文章快速阅读:
.jpg)
文题释义: 促红细胞生成素:是一种可以增加人体血液中红细胞数量、提高血液含氧量的激素,在正常人体内有一定的含量,用于维持和促进正常的红细胞代谢。可以被用来增加贫血患者体内的红细胞数量改善贫血状况。在运动项目中,人为地增加血液中促红细胞生成素的浓度,对提高运动员的运动成绩和提高运动员的耐力,也能够起到一定的作用,因此促红细胞生成素是反兴奋剂检测中最主要的项目之一。 微循环:是指微动脉和微静脉之间的血液循环,是血液与组织细胞进行物质交换的场所。微循环的基本功能是进行血液和组织液之间的物质交换。正常情况下,微循环的血流量与组织器官的代谢水平相适应,保证各组织器官的血液灌流量并调节回心血量。如果微循环发生障碍,将会直接影响各器官的生理功能。
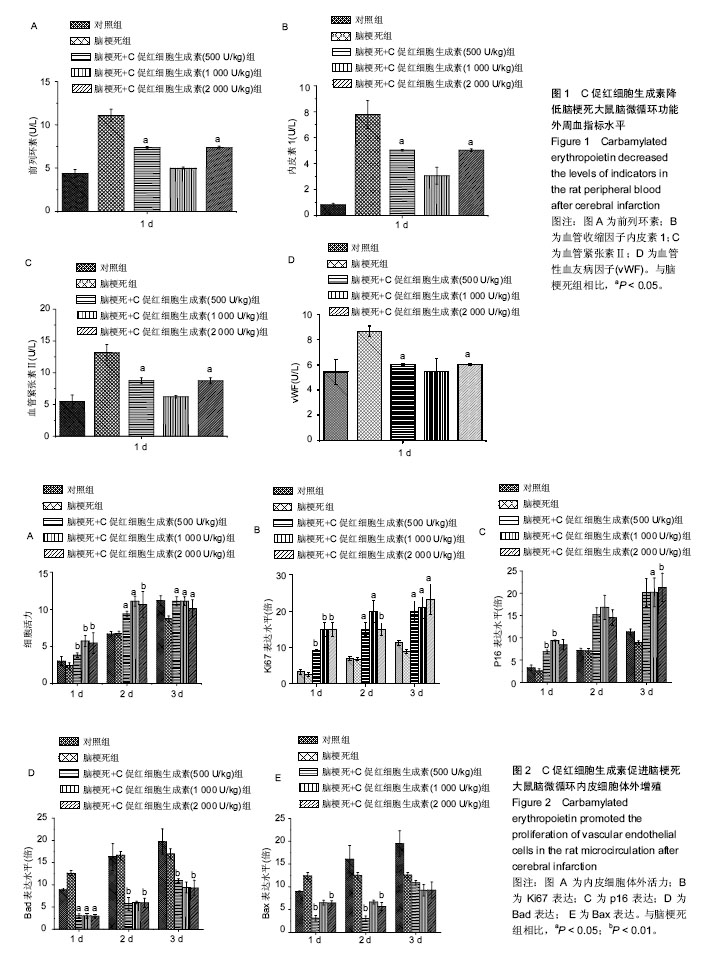
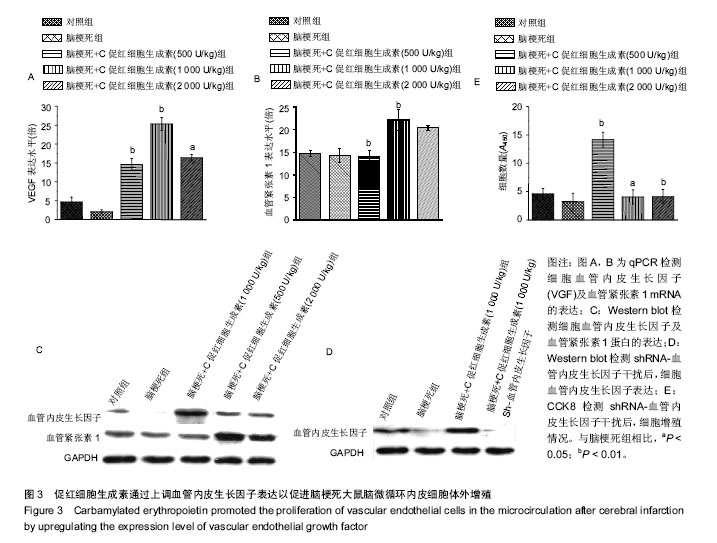
摘要 背景:C促红细胞生成素可明显提升脑梗死患者预后,且促进心肌梗死后脑微循环再生。 目的:探究C促红细胞生成素提升脑梗死患者预后的具体机制。 方法:150只Wistar大鼠,随机取120只建立大鼠脑梗死动物模型后分为:诱导脑梗死组,诱导脑梗死组+C促红细胞生成素处理(浓度500,1 000,2 000 U/kg)组,另30只大鼠为空白对照。体外分离并培养脑梗死动物模型微循环血管内皮细胞,CCK8实验检测细胞增殖情况;Western blot检测与增殖相关基因(Ki67, p16)和血管内皮生长因子蛋白表达,RNA干扰技术选择性沉默血管内皮生长因子蛋白的表达,重复上述指标检测。 结果与结论:①CCK8实验显示,随着C促红细胞生成素浓度的上升,微循环血管内皮细胞的增殖能力也同步上调;Western blot显示,增殖相关基因(Ki67,p16)和血管内皮生长因子蛋白表达水平也显著上调;②shRNA-VEFG 干扰后,CCK8及Western blot实验显示,微循环血管内皮细胞的增殖能力及增殖相关基因(Ki67,p16)的表达未随C促红细胞生成素浓度的上调成正比;③结果提示,C促红细胞生成素通过上调血管内皮生长因子促进脑梗死动物模型微循环血管内皮细胞的增殖。
中图分类号:
.jpg)