中国组织工程研究 ›› 2016, Vol. 20 ›› Issue (37): 5504-5510.doi: 10.3969/j.issn.2095-4344.2016.37.005
• 骨组织构建 bone tissue construction • 上一篇 下一篇
体外震波对骨微血管内皮细胞生理功能的影响
左 伟,高福强,李配瑶,孙 伟,李子荣,时令军
- 中日友好医院骨科,北京市 100029
Impact of extracorporeal shock waves on physiological function of bone microvascular endothelial cells
Zuo Wei, Gao Fu-qiang, Li Pei-yao, Sun Wei, Li Zi-rong, Shi Ling-jun
- Department of Orthopedics, China-Japan Friendship Hospital, Beijing 100029, China
摘要:
文章快速阅读:
.jpg) 文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。
文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。
.jpg) 文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。
文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。摘要
背景:体外震波作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,激活细胞内特殊信号传导系统,调控内皮细胞基因表达,对内皮细胞生理功能产生影响。
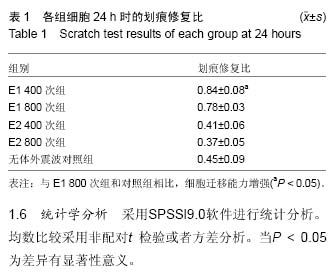
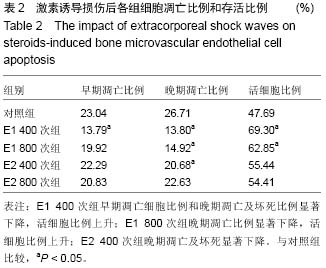
目的:观察不同能流密度及作用次数体外震波对股骨头骨微血管内皮细胞成血管能力、迁移能力及抗凋亡作用的影响。
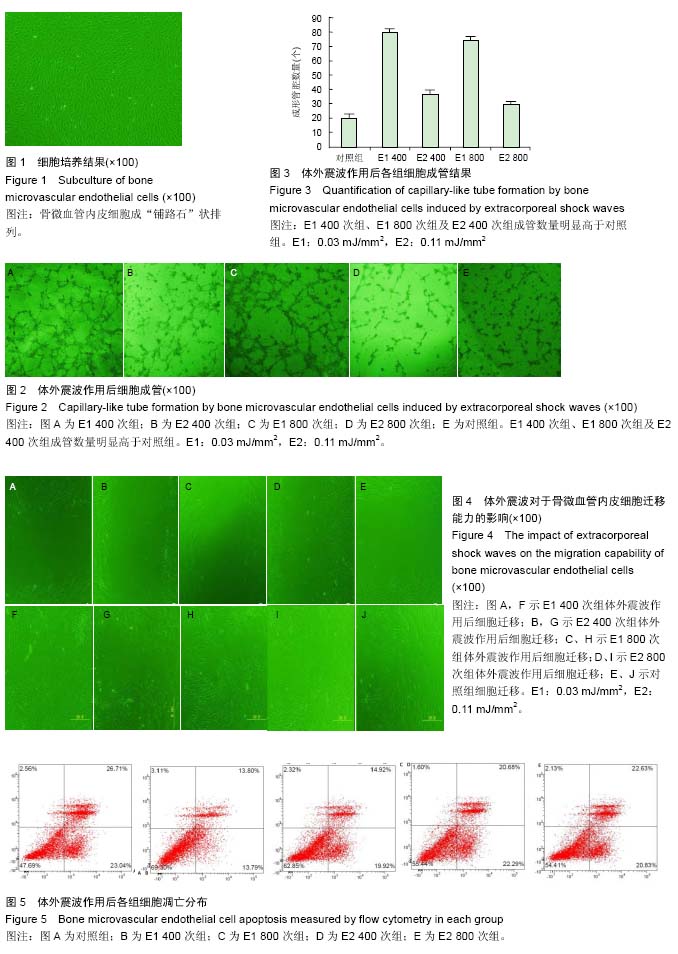
方法:从关节置换患者股骨头获取股骨头骨松质,分离、培养、传代培养股骨头骨微血管内皮细胞。通过血管性血友病因子、CD31血管内皮细胞标记物抗体进行免疫荧光鉴定。根据体外震波不同能流密度(低0.03 mJ/mm2,高0.11 mJ/mm2)及不同作用次数(400次,800次)对细胞进行分组。通过细胞3D培养实验观察内皮细胞成血管能力,划痕实验观察内皮迁移能力,流式细胞仪检测内皮细胞凋亡情况。
结果与结论:①从股骨头松质骨中分离、培养出的细胞均能表达血管性血友病因子、CD31,表明这些细胞具有血管内皮细胞的特征,阳性率接近100%;②体外震波作用于股骨头骨微血管内皮细胞提高其体外成血管能力及迁移能力,低能流密度组促进成血管能力和细胞迁移能力显著优于高能流密度组,高能流密度组内随着作用次数的增加促成血管能力下降;③对于激素诱导股骨头骨微血管内皮细胞凋亡有保护作用;④结果说明,体外震波对股骨头骨微血管内皮细胞生理功能的影响与作用能流密度及作用次数相关。
中国组织工程研究杂志出版内容重点:组织构建;骨细胞;软骨细胞;细胞培养;成纤维细胞;血管内皮细胞;骨质疏松;组织工程
ORCID: 0000-0002-1483-1302(左伟)
中图分类号:
.jpg) 文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。
文题释义:
能流密度:是在一定空间范围内,单位面积(如平方米)所能取得的或单位质量(如kg)能源所能产生的某种能源的能量或功率,是评价能源的主要指标之一。
体外震波:作为一种特殊类型的外源性物理作用信号,通过刺激、激活内皮细胞上机械力学信号感受器,继而进一步激活细胞内特殊信号传导系统,调控内皮细胞基因表达,而对内皮细胞生理功能产生影响。